Consider the differential distillation of Example (6.3. For this system, (n)-heptane with (n)-octane at (1 mathrm{~atm}), the
Question:
Consider the differential distillation of Example (6.3. For this system, \(n\)-heptane with \(n\)-octane at \(1 \mathrm{~atm}\), the average relative volatility is \(\alpha=2.16\) (Treybal, 1980). The mixture will be differentially distilled until the average concentration of the distillate is \(65 \mathrm{~mol} \%\) heptane. Using equation \(6-105\) ) derived in Problem 6.7, compute the composition of the residue, and the fraction of the feed that is distilled.
Data From Example 6.3:-
Suppose the liquid of Example 6.1 [50 mol% n-heptane (A), 50 mol% n-octane (B)] were subjected to a batch distillation at atmospheric pressure, with 60 mol% of the iquid distilled. Compute the composition of the composited distillate and the residue.
Data From Example 6.1:-
A liquid mixture containing 50 mol% n-heptane (A), 50 mol% n-octane (B), at 303 K, is to be continuously flash-vaporized at a pressure of 1 atm to vaporize 60 mol% of the feed. What will be the composition of the vapor and liquid and the temperature in the separator if it behaves as an ideal stage? Calculate the amount of heat to be added per mole of feed. n-Heptane and n-octane form ideal solutions.
Data From Problem 6.7:-
If equation (6-40) describes the equilibrium relation at constant pressure by use of some average relative volatility \(\alpha\) over the concentration range involved, show that equation (6-11) becomes.
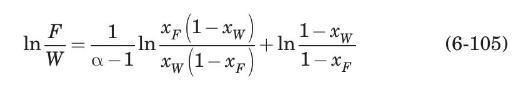
Data From Equation 6-40 and 6-11:-
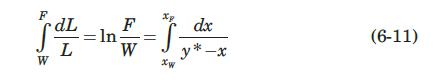

Step by Step Answer:






