Reconsider the balloon discussed in Prob. 1463. Assuming the volume to remain constant and disregarding the diffusion
Question:
Reconsider the balloon discussed in Prob. 14–63. Assuming the volume to remain constant and disregarding the diffusion of air into the balloon, obtain a relation for the variation of pressure in the balloon with time. Using the results obtained and the numerical values given in the problem, determine how long it will take for the pressure inside the balloon to drop to 100 kPa.
Data from problem 63
You probably have noticed that balloons inflated with helium gas rise in the air the first day during a party but they fall down the next day and act like ordinary balloons filled with air. This is because the helium in the balloon slowly leaks out through the wall while air leaks in by diffusion. Consider a balloon that is made of 0.1-mm-thick soft rubber and has a diameter of 15 cm when inflated. The pressure and temperature inside the balloon are initially 110 kPa and 25°C. The permeability of rubber to helium, oxygen, and nitrogen at 25°C are 9.4 × 10-13, 7.05 × 10-13, and 2.6 × 10-13 kmol/m · s · bar, respectively. Determine the initial rates of diffusion of helium, oxygen, and nitrogen through the balloon wall and the mass fraction of helium that escapes the balloon during the first 5 h assuming the helium pressure inside the balloon remains nearly constant. Assume air to be 21 percent oxygen and 79 percent nitrogen by mole numbers and take the room conditions to be 100 kPa and 25°C.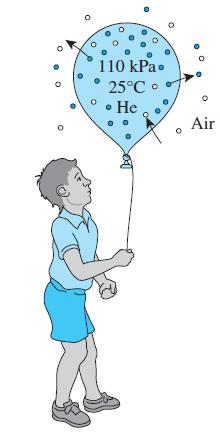
Step by Step Answer:

Heat And Mass Transfer Fundamentals And Applications
ISBN: 9780073398181
5th Edition
Authors: Yunus Cengel, Afshin Ghajar





