The diffusivity of allyl alcohol (left(mathrm{C}_{3} mathrm{H}_{6} mathrm{O} ight)) in a very dilute aqueous solution at (288
Question:
The diffusivity of allyl alcohol \(\left(\mathrm{C}_{3} \mathrm{H}_{6} \mathrm{O}\right)\) in a very dilute aqueous solution at \(288 \mathrm{~K}\) is \(0.9 \times 10^{-5} \mathrm{~cm}^{2} / \mathrm{s}\) (Reid et al., 1987). Based on this result, and the Hayduk and Minhas correlation for aqueous solutions, estimate the molar volume of allyl alcohol at its normal boiling point. Compare to the result obtained using Table 1.2. The viscosity of water at \(288 \mathrm{~K}\) is \(1.15 \mathrm{cP}\).
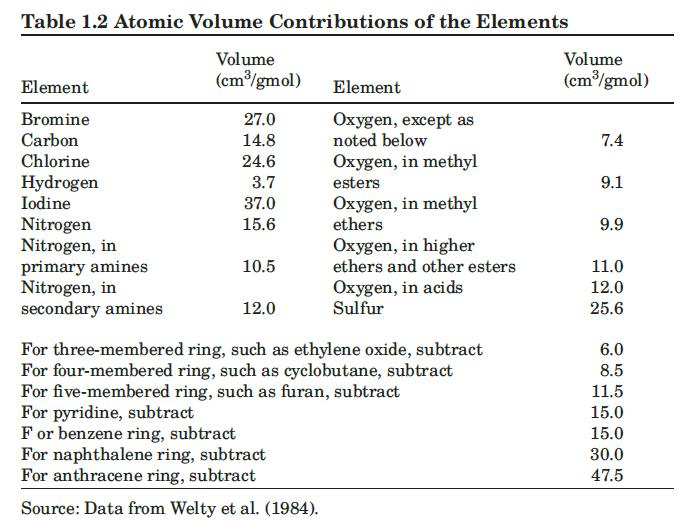
Data From Table 1.2:-
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





