(a) Rationalize why each of the following is diamagnetic: [Os(CN) 6 ] 4 , [PtCl 4 ]...
Question:
(a) Rationalize why each of the following is diamagnetic: [Os(CN)6]4−, [PtCl4]2−, OsO4 and trans-[OsO2F4]2−.
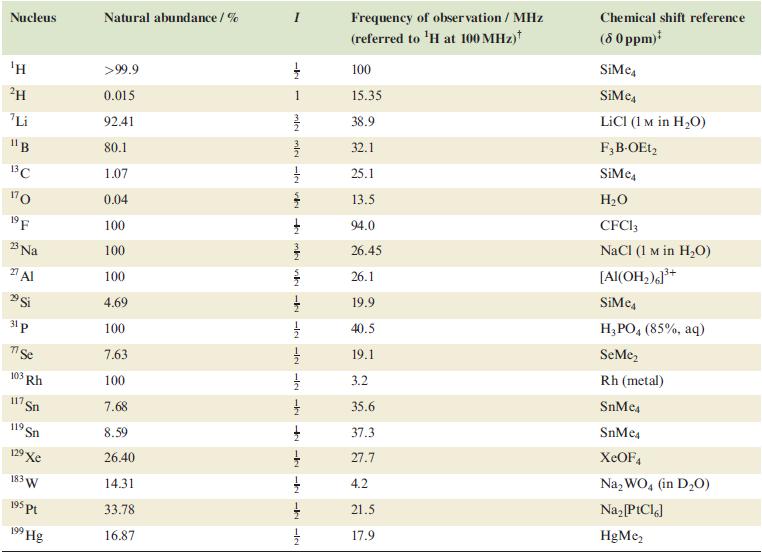
(b) Solution 77Se and 13C NMR spectra for the octahedral anions in the compounds [Bu4N]3[Rh(SeCN)6] and [Bu4N]3[trans-Rh(CN)2(SeCN)4] are tabulated below. Assign the spectra and explain the origin of the observed coupling patterns. [Additional data: see Table 4.3]
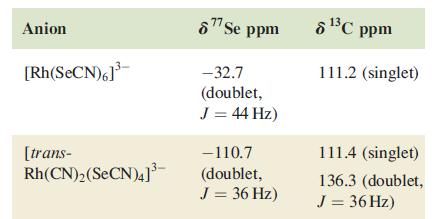
Table 4.3.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





