(a) The reaction of CsF, I 2 O 5 and IF 5 at 435K leads to Cs...
Question:
(a) The reaction of CsF, I2O5 and IF5 at 435K leads to Cs2IOF5. When the amount of CsF is halved, the product is CsIOF4. Write balanced equations for the reactions. Are they redox reactions?
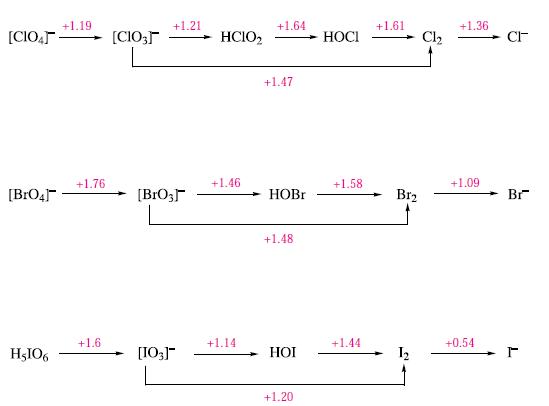
(b) Using data in Fig. 17.14, calculate ΔG°(298 K) for the reaction:
![]()
Comment on the fact that the reaction does not occur at 298 K.
(c) Chlorine dioxide is the major bleaching agent in the pulp industry. While some statistics for bleaching agents list ClO2, others give NaClO3 instead. Suggest reasons for this difference.
Figure 17.14.
Transcribed Image Text:
4[C103] (aq) = 3[C104]¯¯(aq) + Cl¯ (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
a Balanced equations for the reactions Reaction at 435K2 CsF I2O5 5 IF5 Cs2IOF5 When the amount of CsF is halvedCsF I2O5 4 IF5 CsIOF4 2 F2 Regarding w...View the full answer

Answered By

Diana Muriuki
As an online math tutor, I have several years of hands-on experience working with students of all ages and skill levels. I hold a Bachelor's degree in Mathematics and a Master's degree in Education. Additionally, I have completed multiple training courses in online teaching and tutoring methods.
Throughout my career, I have worked with students in both individual and group settings, including classroom teaching, after-school tutoring, and online instruction. I am proficient in teaching a wide range of math topics, from basic arithmetic to advanced calculus and statistics.
One of my greatest strengths as a tutor is my ability to adapt my teaching style to meet the unique needs and learning styles of each individual student. I understand that every student is different, and I strive to create a comfortable and supportive learning environment that encourages growth and development.
In addition to my formal education and tutoring experience, I am also a lifelong learner with a passion for mathematics. I am constantly seeking out new resources and methods to improve my own knowledge and skills, and I believe this passion and enthusiasm helps to inspire my students as well.
Overall, my hands-on experience and proficiency as a math tutor are grounded in a combination of formal education, practical experience, and a genuine love of mathematics. I am confident in my ability to help students achieve their goals and succeed in math, and I look forward to the opportunity to work with new students and continue to grow as an educator.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The Bell Curve (Free Press, 1994), written by Richard Herrnstein and Charles Murray (H&M), is a controversial book about race, genes, IQ, and economic mobility. The book heavily employs statistics...
-
Write balanced equations for the three known reactions that transfer an amino group to a substrate by condensation with aspartate to give an inter-mediate that then undergoes an , -elimination to...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Suppose that In Example 18.6 the electrical firm does not have enough prior information regarding the population mean length of life to be able to assume a normal distribution for p. The firm...
-
Honda Motor Company reports that it has manufacturing facilities in over twenty locations around the world. Only four of those locations are in Japan. Several are in the U.S., Europe, and South...
-
A youngster in a boat on a lake watches waves that seem to be an endless succession of identical crests passing with a half-second interval between each. If every disturbance takes 1.5 s to sweep...
-
Reconsider the data from Problem 51. What is the capital recovery cost of Alternative 3 for a 6-year life? Data from problem 51 Alternatives 1, 2, and 3 have lives of 3, 4, and 6 years, respectively....
-
Consider the unadjusted trial balance of Princess, Inc., at August 31, 2012, and the related month-end adjustment data. Adjustment data at August 31, 2012, include the following: a. Accrued...
-
Determine if the following equations can be solved by separation of variables, and if they are, it solves the problems. 1. 5x7dy+9xy5 = 0 dx 2. -xe +y=0 3. 5xy'-11y = 0 Graph both equations, the...
-
Suggest products for the following (which are not balanced): (a) [CIO3] + Fe+ + H+ (b) [103] + [SO3)- (c) [103] + Br + H+
-
Describe in outline how you would attempt: (a) To determine the equilibrium constant and standard enthalpy change for the aqueous solution reaction: (b) To show that the oxide I 4 O 9 (reported to be...
-
What is the separation basis for absorption? What is the separating agent?
-
ACTIVITY-BASED COSTING, COST DRIVERS Southern Metals Company's controller has established these overhead cost pools and cost drivers for Overhead Cost Pool Machine setups Power Materials handling...
-
Marcel leases a garage. He must pay $300 every week for his lease regardless of how many cars he fixes. The number of cars he fixes each week depends on how many mechanics he hires. The table below...
-
What is the importance of Information system auditing in the financial sector, and how does information system auditing support financial auditing? Use in - text citations, and references and provide...
-
From the following information prepare income statement Revenue 50.000 Salaries 10.000 Rent 10.000 Advertising 10.000 From the following information prepare financial position statement Land 10.000...
-
At the end of the accounting period, Meyer Company has a balance of $ 2 7 , 0 0 0 in the Service Revenue account. What entry should be made to close the Service Revenue account? Select answer from...
-
Last rating period, the percentages of viewers watching several channels between 11P.M. and 11: 30P.M. in a major TV market were as follows: Suppose that in the current rating period, a survey of...
-
If M = 7, s = 2, and X = 9.5, what is z?
-
The rates of H 2 gas absorption (in dm 3 mol 1 s 1 ) by alkenes catalysed by [RhCl(PPh 3 ) 3 ] in benzene at 25C are: hexene, 2910; cis-4-methyl-2-pentene, 990; cyclohexene, 3160;...
-
Infrared spectroscopic investigation of a mixture of CO, H 2 , and 1-butene under conditions that bring about hydroformylation indicate the presence of compound (E) in Fig. 22.20 in the reaction...
-
(a) Starting with the alkene complex shown in Fig. 22.22 with trans-DHC=CHD in place of C 2 H 4 , assume dissolved OH attacks from the side opposite the metal. Give a stereochemical drawing of the...
-
CX Enterprises has the following expected dividends: $1.07 in one year, $1.19 in two years, and $1.35 in three years. After that, its dividends are expected to grow at 4.1% per year forever (so that...
-
Ratios analysis for both Companies comparisons for each year/ quarters, 2019 to 2021 for Current Ratios Quick Ratios Debt to Equity Ratios Debt to capital Asset to equity ratios working capital...
-
Assume Gillette Corporation will pay an annual dividend of $0.68 one year from now. Analysts expect this dividend to grow at 12.8% per year thereafter until the 4th year. Thereafter, growth will...

Study smarter with the SolutionInn App


