Question: (a) Using data from Appendix 6, plot a graph to show how the ionic radii of high-spin, 6-coordinate M 2+ ions of the first row
(a) Using data from Appendix 6, plot a graph to show how the ionic radii of high-spin, 6-coordinate M2+ ions of the first row of the d-block vary with the dn configuration. Comment on factors that contribute to the observed trend.
(b) Briefly discuss other properties of these metal ions that show related trends.
Data from appendix 6.
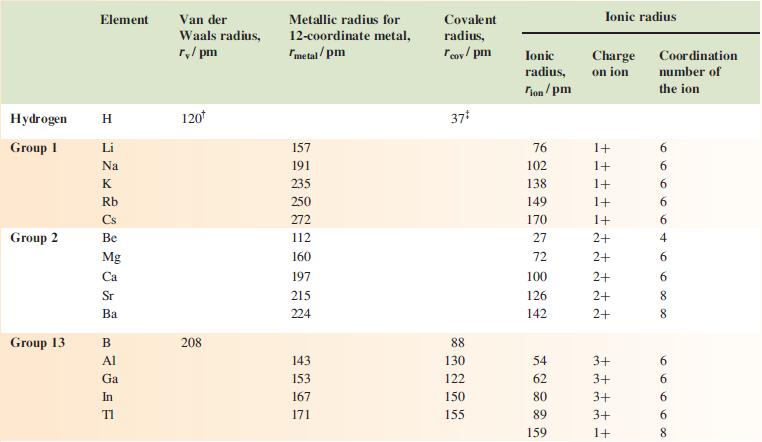
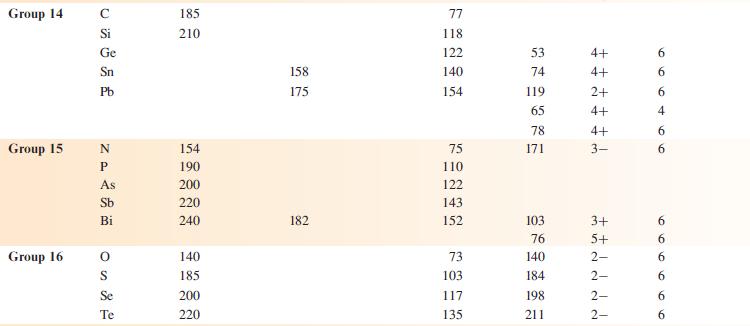
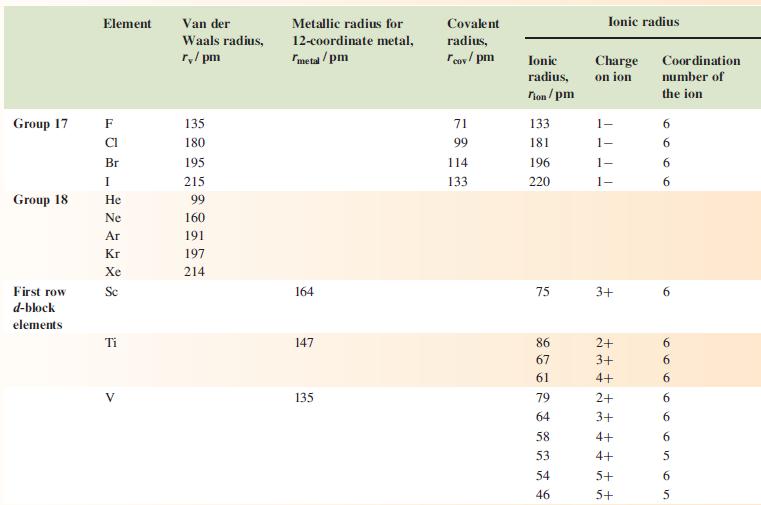
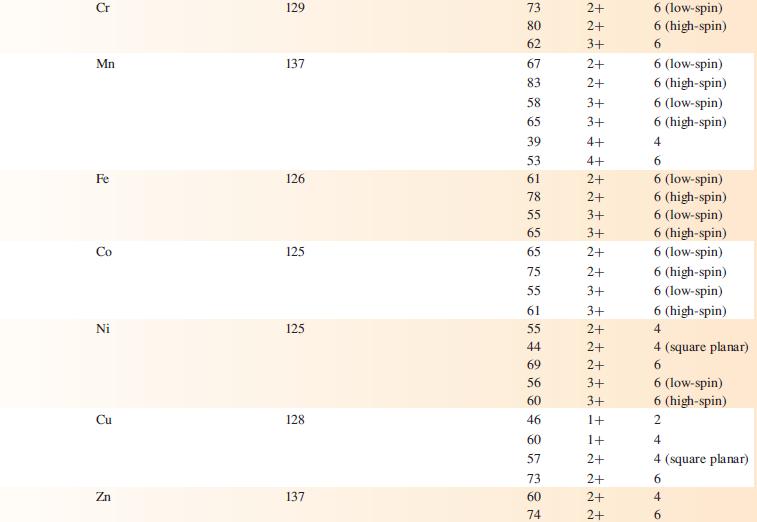
Hydrogen Group 1 Group 2 Group 13 Element H Li Na K Rb Cs Be Mg Ca Sr Ba B Al Ga In TI Van der Waals radius, r/pm 120* 208 Metallic radius for 12-coordinate metal, metal/pm 157 191 235 250 272 112 160 197 215 224 143 153 167 171 Covalent radius, Tov/pm 37* 88 130 122 150 155 Ionic Charge radius, on ion Tion/pm 76 102 138 149 170 27 72 100 126 142 54 62 80 89 159 ++ Ionic radius 1+ 1+ 1+ 1+ 1+ E ++ 2+ 2+ 2+ 2+ d 2+ m m m m - 3+ ++ 3+ 3+ +++ 3+ 1+ Coordination number of the ion 6 6 6 6 6 4 6 100 00 6 8 8 6996 00 8
Step by Step Solution
3.51 Rating (164 Votes )
There are 3 Steps involved in it
a lonic Radii of HighSpin 6Coordinate M lons Variation with dr Configuration To plot a graph showing how the ionic radii of highspin 6coordinate M ions of the first row of the dblock vary with their d ... View full answer

Get step-by-step solutions from verified subject matter experts


