Question: be Why is less heat evolved per mole of Mg+2H+always produces 110kcal/mol magnesium under constant pressure conditions but if any of the H+must be ionized




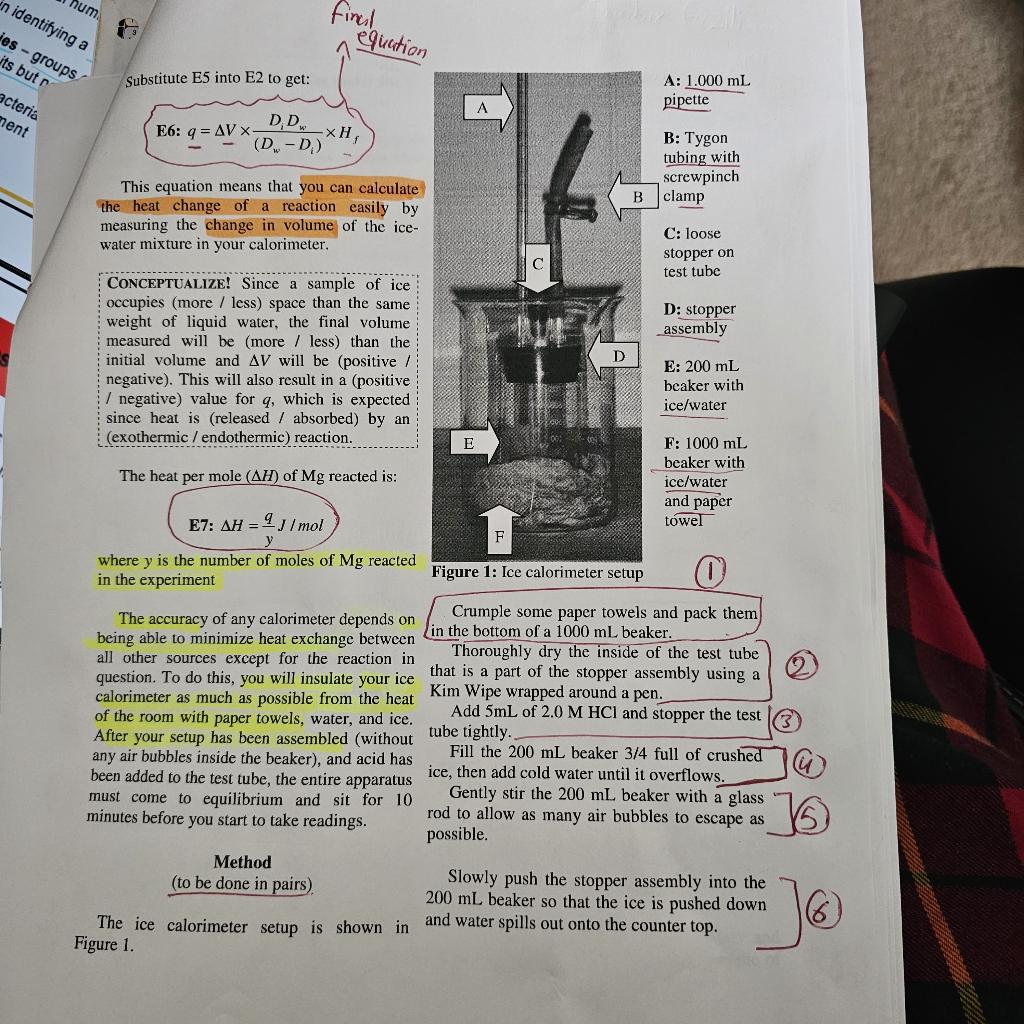

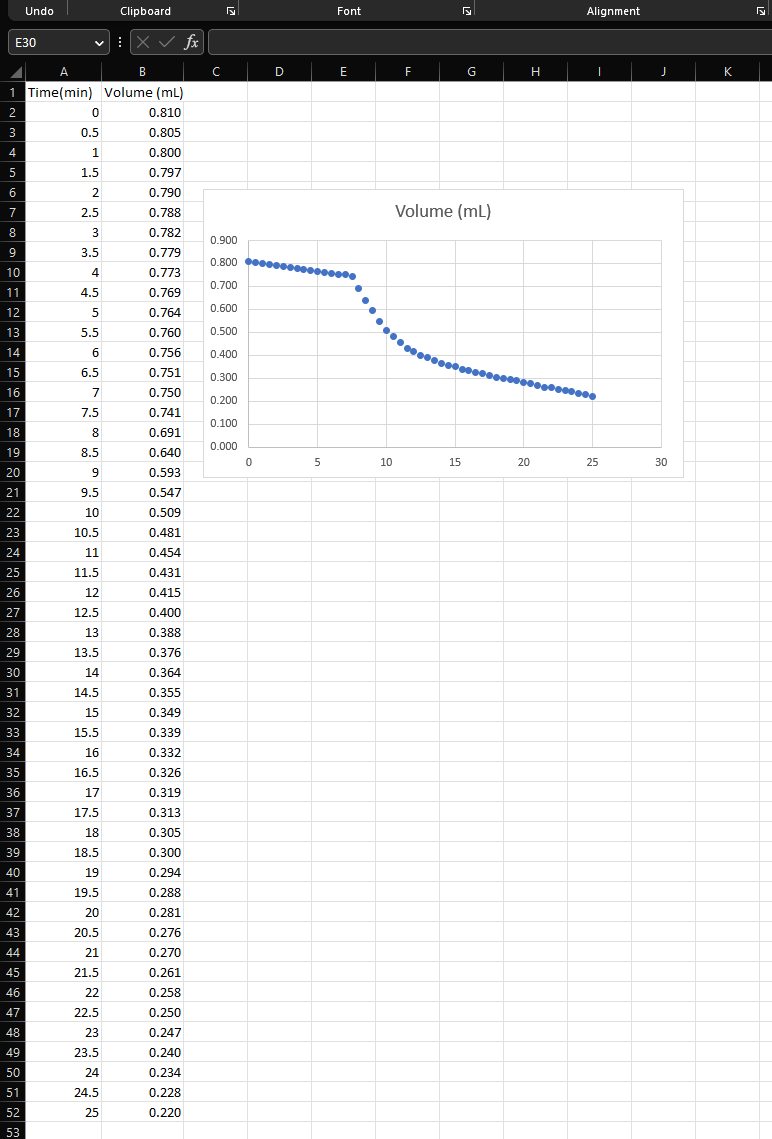


be Why is less heat evolved per mole of Mg+2H+always produces 110kcal/mol magnesium under constant pressure conditions but if any of the H+must be ionized first than under constant volume conditions? In this (which is an endothermic process that uses up reaction, two things happen: heat is given off energy), then less reaction energy is available and H2 gas is formed. to be given off as heat and less heat is measured under these conditions. In other If this reaction was performed in a bomb words, if any endothermic process (such as calorimeter, the volume would remain ionization of an acid or expansion of a gas) constant, the H2 gas could not expand, and all takes place at the same time as an exothermic of the internal energy change produced by the process (such as the reaction of Mg with H+or reaction would be given off as heat. the neutralization of an acid by a base), some (Remember that E for any reaction can be of the energy released by the chemical reaction determined by measuring the heat change per is used up by the endothermic process and thus mole under constant volume conditions.) is not measured as heat. Since this reaction was performed in an ice HCl is a strong acid, which means that it is calorimeter, the pressure remained constant, 100% ionized and does not need to absorb any the H2 gas was allowed to expand (by escaping energy from an exothermic reaction in order from the test tube), and this expansion used up for the H+ions to be available to the Mg.2.0M some of the energy produced by the reaction. HCl provides 2 moles per litre of H+ready to This means that not all of the internal energy react with Mg and this reaction is expected to change was measured as heat under the produce the same amount of heat/mol as shown constant pressure conditions. Thus H will be by the literature value. a smaller negative number than E (i.e. this reaction produces less heat under constant H2SO4 is also a strong acid, but only the pressure conditions than under constant first ionization of H+occurs at 100%; the volume conditions.) This is not true for all second ionization only partially occurs. A 1.0 reactions, so each one must be analyzed M solution of H2SO4 provides about 1.01 separately! mol/L of H+ready to react with Mg and the ions are used up by the reaction. The reaction of Mg with H2SO4 ? The literature released by the Mg and so less energy is the reaction of one mole of Mg with released as heat that can be measured by the calorimeter. This is why the reaction of Mg and H2SO4 is expected to produce less heat per Notice that the chemical equation does not mole of reactant than shown by the literature show the anion of the acid being reacted. This value. means that the anion plays a negligible role in the amount of heat given off by this reaction There are also some experimental sources and it is referred to as a spectator ion. As long of error that would account for H values that as the H+ions are present in solution and ready are different from the literature value for both to react, the same amount of energy will be of the acids used in this experiment. released per mole of Mg reacted, regardless of etween If there are no gases involved in the When expansion work is done, the system paction, or if the moles of gases are the same expends energy by doing work on the on both sides of the equation, then no work can surroundings, and w is given a negative sign. be done regardless of the conditions and When contraction work is done, the H=E. Furthermore, if gases are not surroundings put energy into the system by allowed to expand or contract (i.e. they are doing work on it, and w is given a positive under constant pressure conditions) work still sign. cannot be done. If the reaction is strongly exothermic, both Working with the Equations E and H will be negative. If the reaction is From the textbook, strongly endothermic, both will be positive. E8: E=q+w The sign of the work term where E is the internal energy change during is not determined a reaction, q is the experimental heat, and w is by the signs of E and H. the work done Under constant volume conditions, NoTICE! Use the equation E=H+PV however, no work can be done since V=0. or H=EPV to prove the following This follows that: to yourself: E9:w=PV=0 and E8 can be simplified to: E10:E=qv 1. Exothermic reaction with expansion work: if all three terms are negative, Thus, under constant volume conditions E will be more negative than H (i.e. in a bomb calorimeter), the internal energy and the reaction will give off more change of a chemical reaction is simply the heat under constant volume value of heat measured converted to heat conditions. change per mole of a specified component. 2. Exothermic reaction with contraction Under constant pressure conditions, work work: if E and H are negative and can be done (and is not equal to 0 ). Thus: PV is positive, H will be more negative than E and the reaction will give off more heat under constant pressure conditions. where qp is the heat measured under constant 3. Endothermic reaction with expansion pressure conditions, and may be replaced with work: if E and H are positive and H (after being converted to heat per mole PV is negative, H will be more measured under constant pressure conditions) positive than E and the reaction will This follows that: E12:E=H+PV absorb more heat under constant Thus, under constant pressure conditions pressure conditions. (i.e. in an ice calorimeter), the internal energy change of a chemical reaction is the sum of the 4. Endothermic reaction with enthalpy change and the work done during the contraction work: if all three terms reaction. are positive, E will be more positive than H and the reaction will absorb more heat under constant volume conditions. Details of the Experiment If the rate of room heat melting has not Your graph should end up looking roughly changed during the experiment, a straight line like the one shown in Figure 2 below: have exactly the same slope as the first 14 points. Thus, any vertical distance between these two extrapolated parallel lines is the change in volume (V) due to the reaction, as shown in Figure 2. This graphing method takes any effects from the room temperature environment out of the calculation for V. If the initial and final melt rates are not the same (i.e. the straight lines are not parallel with each other) calculate the V at the t=12 minute mark. Figure 2: Sample graph of pipette volume (mL) vs. time (mins) Thermodynamic Supplement The first 14 volume measurements you took Why do we use two symbols ( H and E) to were to determine the background melting rate designate the heat of a reaction? Many of the ice, caused by a hopefully small but chemical reactions produce a different amount constant absorption of heat from the room. of heat depending on whether they are done Since the temperature of the room is not under constant pressure or constant volume fluctuating wildly, the changes in volume are conditions! expected to be constant and a line of best fit E is the heat change per mole of product should be straight. This background melting produced under constant volume conditions, rate should continue at the same rate even and is referred to as the internal energy while the reaction is taking place. change. H is the heat change per mole of product When the magnesium metal turnings are produced under constant pressure conditions, dropped into the acid, the heat produced is and is referred to as the enthalpychange. greatest at first when there is the most available metal to react. The rate of heat production then The difference in magnitude between E and continues to decrease as the magnesium is used H depends on whether work (w=PV) is up. As the reaction proceeds and the reaction done during the reaction. This kind of work mixture is heated up, the total rate of melting is can only be done if the reaction volume not constant and the line of best fit will be a changes, which is determined by examining smooth curve through the graphed points rather only the gases in a balanced equation. than a straight or zig-zag line. If there are more moles of gaseous products After the reaction is finished and the test than gaseous reactants, there is expansion work tube has cooled back to the initial (nearly 0C ) done (i.e. the reaction volume is larger after the conditions, the absorption of room heat again reaction has taken place). becomes the only reason the ice in the If there are more moles of gaseous reactants calorimeter is melting. than gaseous products, there is contraction work done (i.e. the reaction volume is smaller after the reaction has taken place). Do not trap any air bubbles under the stopper! Take all pipette readings to three significant figures! The first pipette reading should be the largest. (6) With the clamp open, push the stopper assembly firmly into the 200mL beaker until NoTICE! What is the total volume of the water rises into the tubing and remains at a pipette? (7) fairly constant level. If there are large air bubbles trapped under Take a pipette reading at t=, and every the stopper, or the test tube is not completely 30 seconds thereafter for seven minutes, to Woys surrounded by crushed ice, remove the measure the base rate of melting due to the refill the beaker with coldwate and procedure repeat the above proberse this slight decrease in volume until the water level in the tube remains before ever starting an exothermic reaction, but relatively constant. the base rate should remain constant throughout the experiment and not interfere If the water level remains relatively with your results. constant, place the 200mL beaker inside the 1000mL beaker so that the top of the stopper At the seven minute mark, take the pipette assembly is below the lip of the large beaker reading and then drop in the magnesium metal. and pack as many ice cubes around the sides as Loosely replace the small stopper on the test (18) you can fit.] Add water until the top of the tube. (19) stopper assembly is submerged and covered with ice, but the mouth of the test tube remains HYPOTHFSIZE! Why should you not seal the above water. test tube tightly with the small stopper? Use a distilled water bottle to force water Continue to take pipette readings every 30 (i1) no air bubbles!) into the system until the seconds. pipette is filled. (13) Accurately weigh a 0.06-0.08 g sample of pen to push them down. the metal with your fingers! Continue to take pipette readings until the Loosen the small test tube stopper so that rate of volume change returns to the initial base rate you observed (or for about 18 minutes of you and close it easily. you can open and close it easily. Adjust the meniscus so that it is near the top total timing). of the pipette but not past the calibration marks. Rinse the test tube with lots of water and repeat the experiment using another sample of magnesium and 5mL of 1.0MH2SO4. Substitute E5 into E2 to get: E6: q=V(DwDi)DiDwHf This equation means that you can calculate the heat change of a reaction easily by measuring the change in volume of the icewater mixture in your calorimeter. CONCEPTUALIZE! Since a sample of ice occupies (more / less) space than the same weight of liquid water, the final volume measured will be (more / less) than the initial volume and V will be (positive / negative). This will also result in a (positive I negative) value for q, which is expected since heat is (released / absorbed) by an (exothermic / endothermic) reaction. The heat per mole (H) of Mg reacted is: E7: H=yqJ/mol where y is the number of moles of Mg reacted in the experiment The accuracy of any calorimeter depends on Crumple some paper towels and pack them being able to minimize heat exchange between in the bottom of a 1000mL beaker. all other sources except for the reaction in Thoroughly dry the inside of the test tube question. To do this, you will insulate your ice that is a part of the stopper assembly using a (2) calorimeter as much as possible from the heat Kim Wipe wrapped around a pen. of the room with paper towels, water, and ice. Add 5mL of 2.0MHCl and stopper the test After your setup has been assembled (without tube tightly. any air bubbles inside the beaker), and acid has Fill the 200mL beaker 3/4 full of crushed been added to the test tube, the entire apparatus ice, then add cold water until it overflows. must come to equilibrium and sit for 10 Gently stir the 200mL beaker with a glass minutes before you start to take readings. rod to allow as many air bubbles to escape as possible. Method Slowly push the stopper assembly into the (to be done in pairs). 200mL beaker so that the ice is pushed down The ice calorimeter setup is shown in and water spills out onto the counter top. Determining Heat of Reaction: Ice Calorimeter Pre-Lab Readings: Appendix A: Significant Figures Appendix D: Graphing Introduction This means that for every gram of ice melted, 333.5J of heat is absorbed by the ice In this experiment, you will determine the and, therefore, this same amount of heat must amount of heat evolved per mole of be given off by the reaction. magnesium metal when it reacts with an acid according to equation E1 below: Since it would be very difficult to directly the change in volume (caused by the melting APPLY! Why might a chemist want to know ice): the amount of heat given off by a reaction? So we can drive an eigdathcmic E3:V=ViVw recetion to completetisp where Vi is the volume of ice, and Vw is the volume of liquid water (after melting w grams One method to accomplish this is with the ice) use of an ice calorimeter. The simple setup you will use is shown in Figure 1. The reaction Remember that you can relate mass and takes place in a small test tube suspended in a volume by density and derive equation E4: sealed beaker filled with ice and water. As heat given off by an exothermic reaction is absorbed by the surroundings, the ice melts. E4: V=DiwDww Since ice and liquid water have very different where Di is the density of ice at 0C(0.917 densities, melting results in a change in volume g/mL ), and Dw is the density of water at 0C of the ice-water mixture. The heat required to (1.000g/mL) melt a given mass of ice is shown in equation E2: Then rearrange E4 to solve for w (which is E2:q=wHf the unknown term in E2 that you need): where q is heat absorbed by ice, w is mass of ice melted (in grams), and Hf is the latent heat of fusion of ice (333.5J/g) E5:w=V(DwDi)DiDw DATA: Don't forget to attach your data ANALYSIS: 1. On separate sheets, plot volume vs time for each trial. Read the "Detail of the Experiment" section of Experiment 3 in the lab manual to get an idea of what you are graphing. 2. Use Equations (6) and (7) to calculate H for Mg reacting with HCl. Show which points on the graph were used to determine V. Round H to the maximum number of significant figures allowed by your measurements. Weight of Paper 02188 Weight of My 0.0710 be Why is less heat evolved per mole of Mg+2H+always produces 110kcal/mol magnesium under constant pressure conditions but if any of the H+must be ionized first than under constant volume conditions? In this (which is an endothermic process that uses up reaction, two things happen: heat is given off energy), then less reaction energy is available and H2 gas is formed. to be given off as heat and less heat is measured under these conditions. In other If this reaction was performed in a bomb words, if any endothermic process (such as calorimeter, the volume would remain ionization of an acid or expansion of a gas) constant, the H2 gas could not expand, and all takes place at the same time as an exothermic of the internal energy change produced by the process (such as the reaction of Mg with H+or reaction would be given off as heat. the neutralization of an acid by a base), some (Remember that E for any reaction can be of the energy released by the chemical reaction determined by measuring the heat change per is used up by the endothermic process and thus mole under constant volume conditions.) is not measured as heat. Since this reaction was performed in an ice HCl is a strong acid, which means that it is calorimeter, the pressure remained constant, 100% ionized and does not need to absorb any the H2 gas was allowed to expand (by escaping energy from an exothermic reaction in order from the test tube), and this expansion used up for the H+ions to be available to the Mg.2.0M some of the energy produced by the reaction. HCl provides 2 moles per litre of H+ready to This means that not all of the internal energy react with Mg and this reaction is expected to change was measured as heat under the produce the same amount of heat/mol as shown constant pressure conditions. Thus H will be by the literature value. a smaller negative number than E (i.e. this reaction produces less heat under constant H2SO4 is also a strong acid, but only the pressure conditions than under constant first ionization of H+occurs at 100%; the volume conditions.) This is not true for all second ionization only partially occurs. A 1.0 reactions, so each one must be analyzed M solution of H2SO4 provides about 1.01 separately! mol/L of H+ready to react with Mg and the ions are used up by the reaction. The reaction of Mg with H2SO4 ? The literature released by the Mg and so less energy is the reaction of one mole of Mg with released as heat that can be measured by the calorimeter. This is why the reaction of Mg and H2SO4 is expected to produce less heat per Notice that the chemical equation does not mole of reactant than shown by the literature show the anion of the acid being reacted. This value. means that the anion plays a negligible role in the amount of heat given off by this reaction There are also some experimental sources and it is referred to as a spectator ion. As long of error that would account for H values that as the H+ions are present in solution and ready are different from the literature value for both to react, the same amount of energy will be of the acids used in this experiment. released per mole of Mg reacted, regardless of etween If there are no gases involved in the When expansion work is done, the system paction, or if the moles of gases are the same expends energy by doing work on the on both sides of the equation, then no work can surroundings, and w is given a negative sign. be done regardless of the conditions and When contraction work is done, the H=E. Furthermore, if gases are not surroundings put energy into the system by allowed to expand or contract (i.e. they are doing work on it, and w is given a positive under constant pressure conditions) work still sign. cannot be done. If the reaction is strongly exothermic, both Working with the Equations E and H will be negative. If the reaction is From the textbook, strongly endothermic, both will be positive. E8: E=q+w The sign of the work term where E is the internal energy change during is not determined a reaction, q is the experimental heat, and w is by the signs of E and H. the work done Under constant volume conditions, NoTICE! Use the equation E=H+PV however, no work can be done since V=0. or H=EPV to prove the following This follows that: to yourself: E9:w=PV=0 and E8 can be simplified to: E10:E=qv 1. Exothermic reaction with expansion work: if all three terms are negative, Thus, under constant volume conditions E will be more negative than H (i.e. in a bomb calorimeter), the internal energy and the reaction will give off more change of a chemical reaction is simply the heat under constant volume value of heat measured converted to heat conditions. change per mole of a specified component. 2. Exothermic reaction with contraction Under constant pressure conditions, work work: if E and H are negative and can be done (and is not equal to 0 ). Thus: PV is positive, H will be more negative than E and the reaction will give off more heat under constant pressure conditions. where qp is the heat measured under constant 3. Endothermic reaction with expansion pressure conditions, and may be replaced with work: if E and H are positive and H (after being converted to heat per mole PV is negative, H will be more measured under constant pressure conditions) positive than E and the reaction will This follows that: E12:E=H+PV absorb more heat under constant Thus, under constant pressure conditions pressure conditions. (i.e. in an ice calorimeter), the internal energy change of a chemical reaction is the sum of the 4. Endothermic reaction with enthalpy change and the work done during the contraction work: if all three terms reaction. are positive, E will be more positive than H and the reaction will absorb more heat under constant volume conditions. Details of the Experiment If the rate of room heat melting has not Your graph should end up looking roughly changed during the experiment, a straight line like the one shown in Figure 2 below: have exactly the same slope as the first 14 points. Thus, any vertical distance between these two extrapolated parallel lines is the change in volume (V) due to the reaction, as shown in Figure 2. This graphing method takes any effects from the room temperature environment out of the calculation for V. If the initial and final melt rates are not the same (i.e. the straight lines are not parallel with each other) calculate the V at the t=12 minute mark. Figure 2: Sample graph of pipette volume (mL) vs. time (mins) Thermodynamic Supplement The first 14 volume measurements you took Why do we use two symbols ( H and E) to were to determine the background melting rate designate the heat of a reaction? Many of the ice, caused by a hopefully small but chemical reactions produce a different amount constant absorption of heat from the room. of heat depending on whether they are done Since the temperature of the room is not under constant pressure or constant volume fluctuating wildly, the changes in volume are conditions! expected to be constant and a line of best fit E is the heat change per mole of product should be straight. This background melting produced under constant volume conditions, rate should continue at the same rate even and is referred to as the internal energy while the reaction is taking place. change. H is the heat change per mole of product When the magnesium metal turnings are produced under constant pressure conditions, dropped into the acid, the heat produced is and is referred to as the enthalpychange. greatest at first when there is the most available metal to react. The rate of heat production then The difference in magnitude between E and continues to decrease as the magnesium is used H depends on whether work (w=PV) is up. As the reaction proceeds and the reaction done during the reaction. This kind of work mixture is heated up, the total rate of melting is can only be done if the reaction volume not constant and the line of best fit will be a changes, which is determined by examining smooth curve through the graphed points rather only the gases in a balanced equation. than a straight or zig-zag line. If there are more moles of gaseous products After the reaction is finished and the test than gaseous reactants, there is expansion work tube has cooled back to the initial (nearly 0C ) done (i.e. the reaction volume is larger after the conditions, the absorption of room heat again reaction has taken place). becomes the only reason the ice in the If there are more moles of gaseous reactants calorimeter is melting. than gaseous products, there is contraction work done (i.e. the reaction volume is smaller after the reaction has taken place). Do not trap any air bubbles under the stopper! Take all pipette readings to three significant figures! The first pipette reading should be the largest. (6) With the clamp open, push the stopper assembly firmly into the 200mL beaker until NoTICE! What is the total volume of the water rises into the tubing and remains at a pipette? (7) fairly constant level. If there are large air bubbles trapped under Take a pipette reading at t=, and every the stopper, or the test tube is not completely 30 seconds thereafter for seven minutes, to Woys surrounded by crushed ice, remove the measure the base rate of melting due to the refill the beaker with coldwate and procedure repeat the above proberse this slight decrease in volume until the water level in the tube remains before ever starting an exothermic reaction, but relatively constant. the base rate should remain constant throughout the experiment and not interfere If the water level remains relatively with your results. constant, place the 200mL beaker inside the 1000mL beaker so that the top of the stopper At the seven minute mark, take the pipette assembly is below the lip of the large beaker reading and then drop in the magnesium metal. and pack as many ice cubes around the sides as Loosely replace the small stopper on the test (18) you can fit.] Add water until the top of the tube. (19) stopper assembly is submerged and covered with ice, but the mouth of the test tube remains HYPOTHFSIZE! Why should you not seal the above water. test tube tightly with the small stopper? Use a distilled water bottle to force water Continue to take pipette readings every 30 (i1) no air bubbles!) into the system until the seconds. pipette is filled. (13) Accurately weigh a 0.06-0.08 g sample of pen to push them down. the metal with your fingers! Continue to take pipette readings until the Loosen the small test tube stopper so that rate of volume change returns to the initial base rate you observed (or for about 18 minutes of you and close it easily. you can open and close it easily. Adjust the meniscus so that it is near the top total timing). of the pipette but not past the calibration marks. Rinse the test tube with lots of water and repeat the experiment using another sample of magnesium and 5mL of 1.0MH2SO4. Substitute E5 into E2 to get: E6: q=V(DwDi)DiDwHf This equation means that you can calculate the heat change of a reaction easily by measuring the change in volume of the icewater mixture in your calorimeter. CONCEPTUALIZE! Since a sample of ice occupies (more / less) space than the same weight of liquid water, the final volume measured will be (more / less) than the initial volume and V will be (positive / negative). This will also result in a (positive I negative) value for q, which is expected since heat is (released / absorbed) by an (exothermic / endothermic) reaction. The heat per mole (H) of Mg reacted is: E7: H=yqJ/mol where y is the number of moles of Mg reacted in the experiment The accuracy of any calorimeter depends on Crumple some paper towels and pack them being able to minimize heat exchange between in the bottom of a 1000mL beaker. all other sources except for the reaction in Thoroughly dry the inside of the test tube question. To do this, you will insulate your ice that is a part of the stopper assembly using a (2) calorimeter as much as possible from the heat Kim Wipe wrapped around a pen. of the room with paper towels, water, and ice. Add 5mL of 2.0MHCl and stopper the test After your setup has been assembled (without tube tightly. any air bubbles inside the beaker), and acid has Fill the 200mL beaker 3/4 full of crushed been added to the test tube, the entire apparatus ice, then add cold water until it overflows. must come to equilibrium and sit for 10 Gently stir the 200mL beaker with a glass minutes before you start to take readings. rod to allow as many air bubbles to escape as possible. Method Slowly push the stopper assembly into the (to be done in pairs). 200mL beaker so that the ice is pushed down The ice calorimeter setup is shown in and water spills out onto the counter top. Determining Heat of Reaction: Ice Calorimeter Pre-Lab Readings: Appendix A: Significant Figures Appendix D: Graphing Introduction This means that for every gram of ice melted, 333.5J of heat is absorbed by the ice In this experiment, you will determine the and, therefore, this same amount of heat must amount of heat evolved per mole of be given off by the reaction. magnesium metal when it reacts with an acid according to equation E1 below: Since it would be very difficult to directly the change in volume (caused by the melting APPLY! Why might a chemist want to know ice): the amount of heat given off by a reaction? So we can drive an eigdathcmic E3:V=ViVw recetion to completetisp where Vi is the volume of ice, and Vw is the volume of liquid water (after melting w grams One method to accomplish this is with the ice) use of an ice calorimeter. The simple setup you will use is shown in Figure 1. The reaction Remember that you can relate mass and takes place in a small test tube suspended in a volume by density and derive equation E4: sealed beaker filled with ice and water. As heat given off by an exothermic reaction is absorbed by the surroundings, the ice melts. E4: V=DiwDww Since ice and liquid water have very different where Di is the density of ice at 0C(0.917 densities, melting results in a change in volume g/mL ), and Dw is the density of water at 0C of the ice-water mixture. The heat required to (1.000g/mL) melt a given mass of ice is shown in equation E2: Then rearrange E4 to solve for w (which is E2:q=wHf the unknown term in E2 that you need): where q is heat absorbed by ice, w is mass of ice melted (in grams), and Hf is the latent heat of fusion of ice (333.5J/g) E5:w=V(DwDi)DiDw DATA: Don't forget to attach your data ANALYSIS: 1. On separate sheets, plot volume vs time for each trial. Read the "Detail of the Experiment" section of Experiment 3 in the lab manual to get an idea of what you are graphing. 2. Use Equations (6) and (7) to calculate H for Mg reacting with HCl. Show which points on the graph were used to determine V. Round H to the maximum number of significant figures allowed by your measurements. Weight of Paper 02188 Weight of My 0.0710
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


