Comment on each of the following observations. (a) AlF 3 is almost insoluble in anhydrous HF, but
Question:
Comment on each of the following observations.
(a) AlF3 is almost insoluble in anhydrous HF, but dissolves if KF is present. Passage of BF3 through the resulting solution causes AlF3 to reprecipitate.
(b) The Raman spectra of germanium tetrachloride, a solution of gallium trichloride in concentrated hydrochloric acid, and fused gallium dichloride contain the following lines:
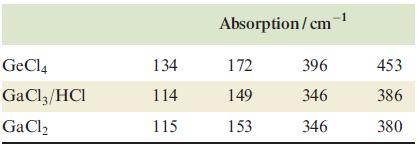
(c) When TlI3, which is isomorphous with the alkali metal triiodides, is treated with aqueous NaOH, hydrated Tl2O3 is quantitatively precipitated.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





