Data from Tables 6.3 and 6.4 are needed for this problem. (a) Estimate the lattice energy of
Question:
Data from Tables 6.3 and 6.4 are needed for this problem.
(a) Estimate the lattice energy of CsCl if the Cs—Cl internuclear distance is 356.6 pm.
(b) Now consider a polymorph of CsCl that crystallizes with an NaCl structure. Estimate its lattice energy given that the Cs—Cl distance is 347.4 pm.
(c) What conclusions can you draw from your answers to parts (a) and (b)?
Table 6.3
Values of the Born exponent, n, given for an ionic compound MX in terms of the electronic configuration of the ions [M+][X‾]. The value of n for an ionic compound is determined by averaging the component values, e.g. for MgO, n = 7; for LiCl
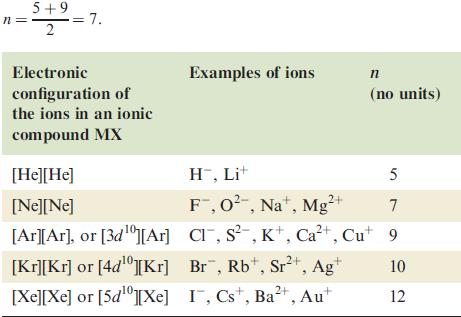
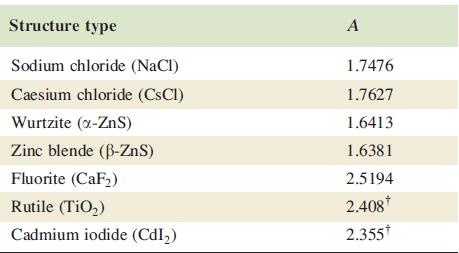
Table 6.4
Madelung constants, A, for selected structure types. Values of A are numerical and have no units.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





