Question: Given the following standard potentials in basic solution and assuming that a reversible reaction can be established on a suitable catalyst, calculate E ,
Given the following standard potentials in basic solution
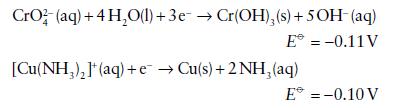
and assuming that a reversible reaction can be established on a suitable catalyst, calculate E⦵, ΔrG⦵, and K for the reductions of
(a) CrO42−
(b) [Cu(NH3)2]+ in basic solution. Comment on why ΔrG⦵ and K are so different between the two cases despite the values of E⦵ being so similar.
CrO2 (aq) + 4HO(l) + 3e Cr(OH), (s) + 5OH- (aq) E = -0.11 V [Cu(NH)](aq) + eCu(s) + 2NH (aq) E* = -0.10 V
Step by Step Solution
3.36 Rating (159 Votes )
There are 3 Steps involved in it
To calculate E standard cell potential rG standard Gibbs free energy change and K equilibrium constant for the reductions of CrO42 and CuNH32 in basic ... View full answer

Get step-by-step solutions from verified subject matter experts


