In vehicles, the combustion of H 2 rather than a hydrocarbon-based fuel reduces both CO 2 emissions
Question:
In vehicles, the combustion of H2 rather than a hydrocarbon-based fuel reduces both CO2 emissions and a dependence on fossil fuels.
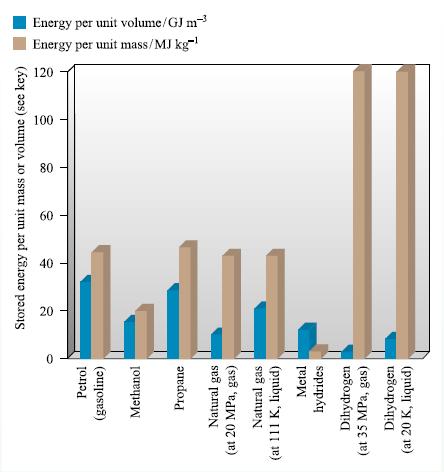
(a) Using data from Appendix 12, show that the combustion of H2 releases 120 kJ g−1.
(b) The chart in Box 10.2 shows that the stored energy per unit mass of compressed (at 35 MPa) H2 gas and of liquid H2 are the same, but that liquid H2 stores more energy per unit volume than compressed H2. Rationalize these data.
(c) 3 kg of gasoline (petrol) is equivalent to 1kg of H2 in terms of stored energy. Comment on this fact in terms of the practical application of H2 as a fuel in a family saloon car.
Data from Appendix 12
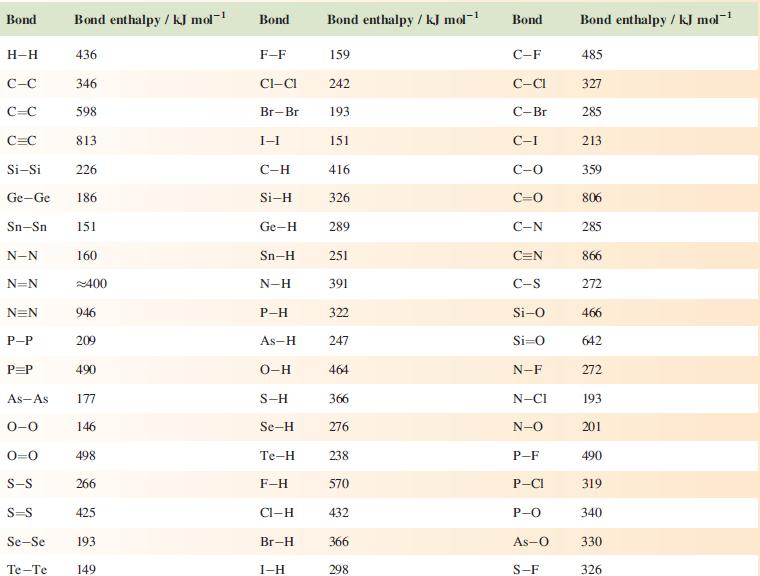
Chart from Box 10.2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





