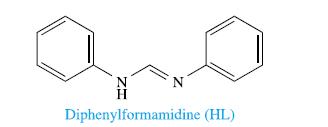
The complex [V 2 L 4 ], where HL is diphenylformamidine (see structure opposite-above), is diamagnetic. Each
Question:
The complex [V2L4], where HL is diphenylformamidine (see structure opposite-above), is diamagnetic. Each L− ligand acts as a bridging, N;N'-donor such that the complex is structurally similar to complexes of the type [Cr2(O2CR)4].
(a) Describe a bonding scheme for the [V2]4+ core and derive the formal metal–metal bond order in [V2L4].
(b) The reaction of [V2L4] with KC8 in THF results in the formation of K(THF)3[V2L4]. What is the role of KC8 in this reaction?
(c) Do you expect the VV bond length to increase or decrease on going from [V2L4] to K(THF)3[V2L4]? Rationalize your answer.
Structure
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:




