The compound shown below is a formazan dye usually referred to as zincon. It is used to
Question:
The compound shown below is a formazan dye usually referred to as ‘zincon’. It is used to detect Zn2+ and Cu2+ ions:
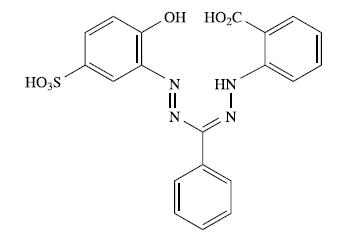
(a) Suggest how the ligand binds to Zn2+ or Cu2+, and comment on the role of pH in determining the overall charge of the complex.
(b) Why does the ligand include a SO3H substituent?
(c) Zincon itself absorbs at 463 nm. Suggest how the absorption arises.
(d) When zincon binds Cu2+, the absorption at 463 nm is replaced by one at 600 nm. Why does this make zincon an easy method of detection for Cu2+ ions?
(e) The copper(II) complex of zincon can be used as a sensor for [CN]− ions in aqueous solution. Addition of [CN]− results in the disappearance of the absorption at 600 nm and reappearance of the absorption at 463 nm. Outline the chemical changes occurring in solution.
Step by Step Answer:






