Question:
The existence of a maximum oxidation number of +6 for both uranium (Z = 92) and tungsten (Z = 74) prompted the placement of U under W in early periodic tables. When the element after uranium, neptunium (Z = 93), was discovered in 1940, its properties did not correspond to those of rhenium (Z = 75), and this cast doubt on the original placement of uranium. Using standard potential data from Resource section 2, discuss the differences in oxidation state stability between Np and Re.
Data from Resource section 2.
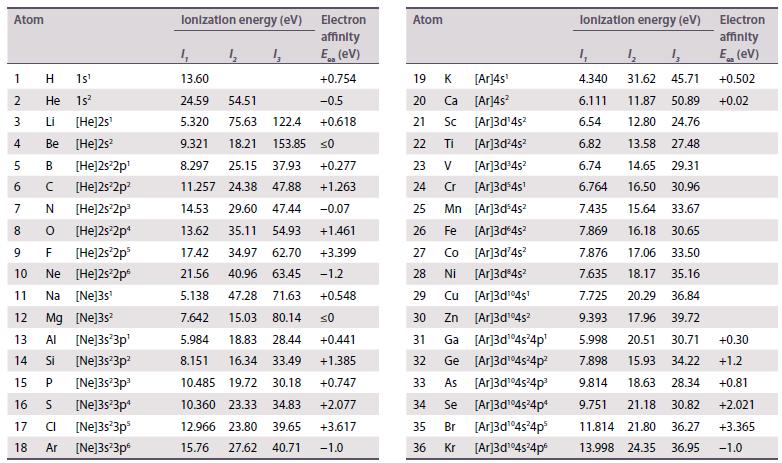
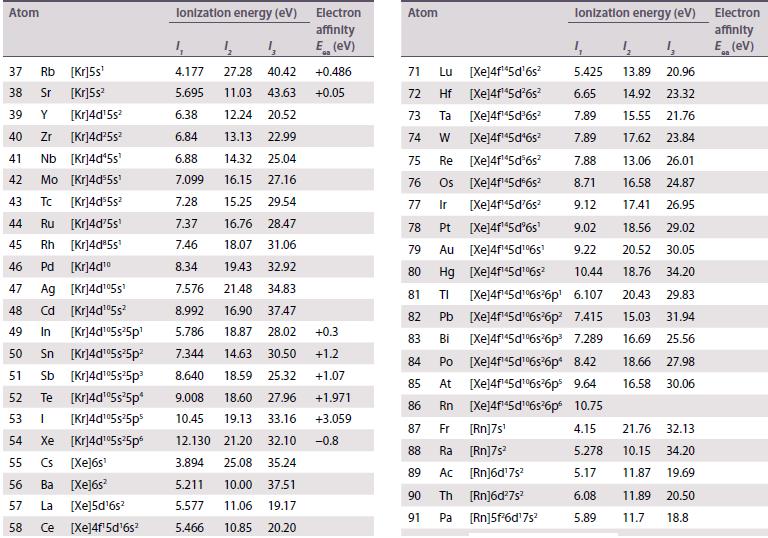
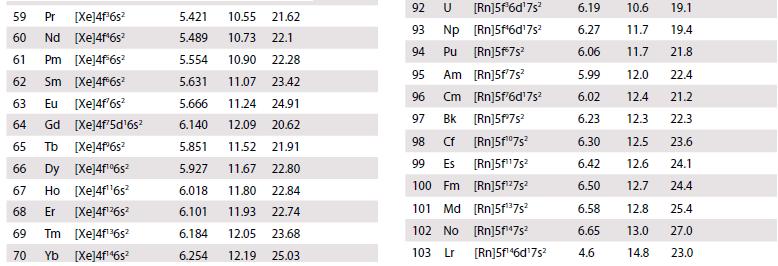
Transcribed Image Text:
Atom
1
H
1s¹
2 He
1s²
3
Li
[He]2s¹
4
Be [He]2s²
5 B
[He]2s²2p¹
6
C
[He]2s²2p²
7
N [He]2s²2p³
8
0
[He]2s²2p¹
9
F
[He]2s²2p³
10 Ne [He]2s²2p
11 Na [Ne]3s¹
12 Mg [Ne]3s²
13 Al [Ne]3s²3p¹
14 Si [Ne]3s²3p²
15 P [Ne]3s¹3p¹
16 S
[Ne]3s²3p¹
17 CI
[Ne]3s 3ps
[Ne]3s²3p6
16
18 Ar
Ionization energy (ev)
4
4₂
Electron
affinity
E (ev)
4
13.60
24.59 54.51
5.320 75.63 122.4
9.321 18.21
153.85
8.297 25.15 37.93 +0.277
11.257 24.38 47.88 +1.263
14.53 29.60 47.44 -0.07
13.62
35.11 54.93 +1.461
17.42 34.97 62.70 +3.399
21.56 40.96 63.45 -1.2
5.138 47.28 71.63 +0.548
7.642 15.03 80.14 ≤0
5.984 18.83 28.44 +0.441
8.151 16.34 33.49 +1.385
10.485 19.72 30.18 +0.747
10.360 23.33 34.83 +2.077
+3.617
-1.0
12.966 23.80 39.65
15.76 27.62 40.71
+0.754
-0.5
+0.618
0
Atom
19 K
[Ar]4s¹
20 Ca
[Ar]4s²
[Ar]3d¹4s²
21 Sc
22 Ti
[Ar]3d²4s²
23 V
[Ar]3d³4s²
24 Cr
[Ar]3d³4s¹
25 Mn [Ar]3d³4s²
26 Fe [Ar]3d 4s²
27 Co [Ar]3d²4s²
28 Ni [Ar]3d³4s²
29 Cu [Ar]3d¹04s¹
30 Zn [Ar]3d¹04s²
31 Ga [Ar]3d¹4s²4p¹
32 Ge [Ar]3d¹04s²4p²
33 As [Ar]3d¹04s²4p³
34 Se [Ar]3d¹04s²4pª
35 Br [Ar]3d¹04s²4p³
36 Kr [Ar]3d¹04s²4p6
Ionization energy (eV)
13
31.62 45.71
11.87 50.89
12.80 24.76
1₁
4.340
6.111
6.54
6.82
13.58 27.48
6.74
14.65 29.31
6.764
16.50 30.96
7.435
15.64
33.67
7.869 16.18 30.65
7.876 17.06 33.50
7.635 18.17 35.16
7.725 20.29 36.84
9.393 17.96 39.72
5.998 20.51 30.71
7.898 15.93 34.22
9.814 18.63 28.34
9.751 21.18 30.82
11.814 21.80 36.27
13.998 24.35 36.95
Electron
affinity
E (EV)
+0.502
+0.02
+0.30
+1.2
+0.81
+2.021
+3.365
-1.0










