The UV-VIS spectrum of a CH 2 Cl 2 solution of the gold (I) compound shown below
Question:
The UV-VIS spectrum of a CH2Cl2 solution of the gold (I) compound shown below with R = Ph is: λmax (ε) = 239 (92500), 269 (67 000), 286 (72 000), 303 (28 000), 315 nm (21000 dm3 mol−1 cm−1).
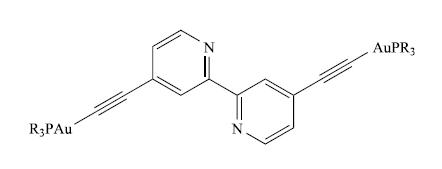
(a) π* ← π transitions contribute to the observed spectrum. How do these arise?
(b) Is the compound coloured?
(c) You are asked to compare the UV-VIS spectra of a series of these compounds with different R substituents. Why should you compare plots of ε against λ rather than A against λ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





