Question: Use data from Appendix 11 to predict qualitatively the outcome of the following experiment at 298 K:Cr is dissolved in excess of molar HClO 4
Use data from Appendix 11 to predict qualitatively the outcome of the following experiment at 298 K:Cr is dissolved in excess of molar HClO4 and the solution is shaken in air.
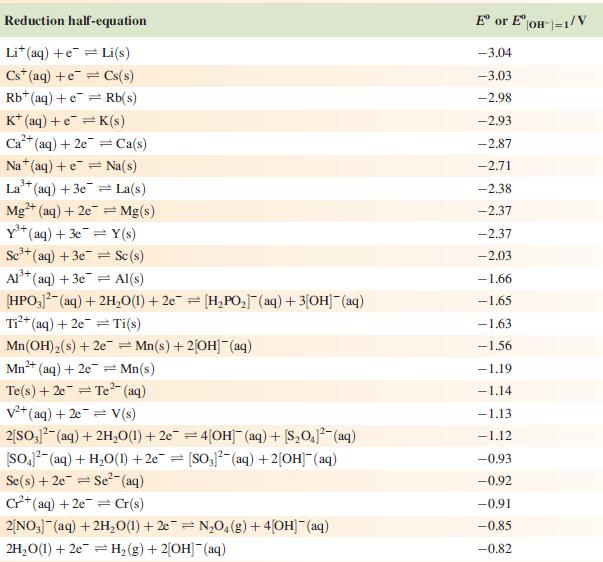
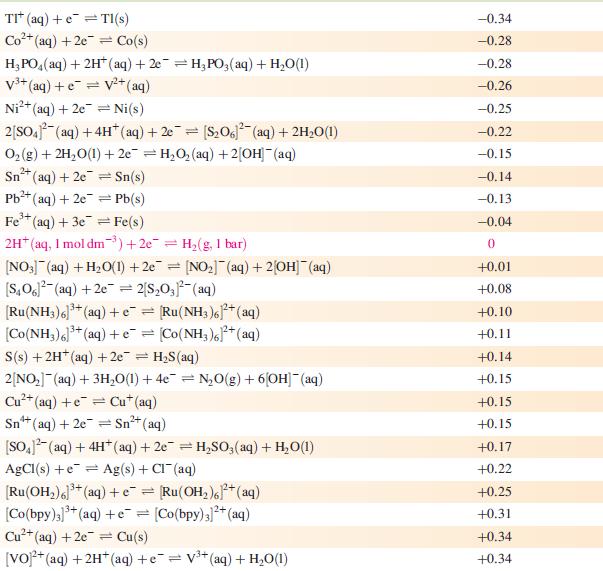
Data from Appendix 11
The concentration of each aqueous solution is 1 mol dm−3 and the pressure of a gaseous component is 1 bar (105 Pa). (Changing the standard pressure to 1 atm (101 300 Pa) makes no difference to the values of Eo at this level of accuracy.) Each half-cell listed contains the specified solution species at a concentration of 1 mol dm−3; where the half-cell contains [OH]−, the value of Eo refers to [OH−] = 1 mol dm−3, hence the notation Eo[OH−] = 1

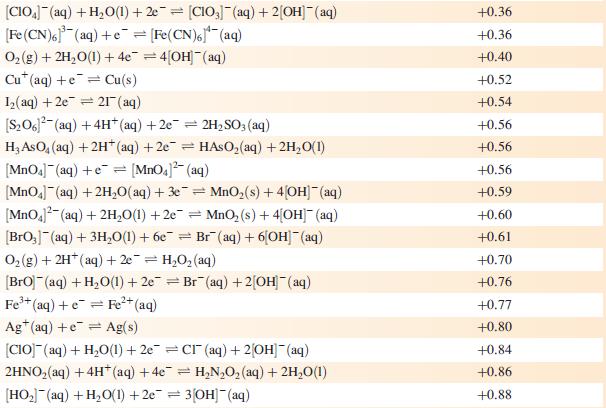
![Al(s) Al+ (aq) + 3e [HPO3)(aq) + 2HO(1) + 2e [HPO] (aq)](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2023/07/64c0cad41904f_43564c0cad382ac9.jpg)

Reduction half-equation Li (aq) +eLi(s) Cst (aq) +eCs(s) Rb+ (aq) + e Rb(s) K+ (aq) + eK(s) 2+ Ca+ (aq) + 2e Ca(s) Na (aq) + e Na(s) 3+ La+ (aq) + 3e = 2+ Mg+ (aq) + 2e Y+ (aq) + 3eY(s) Se+ (aq) + 3e Sc(s) Al(s) Al+ (aq) + 3e [HPO3)(aq) + 2HO(1) + 2e [HPO] (aq) + 3[OH(aq) = = La(s) = Mg(s) Ti+ (aq) + 2e Ti(s) Mn(OH) (s) +2e=Mn(s) + 2OH(aq) Mn+ (aq) + 2e = Mn(s) 2+ Te(s) + 2e Te (aq) = = V+ (aq) + 2e = V(s) 2[SO3)(aq) + 2HO(1) +2e=4[OH] (aq) + [S01(aq) [SO4- (aq) + HO(1) +2e= [SO3)2(aq) + 2[OH] (aq) Se(s) + 2e Se- (aq) C+ (aq) +2e Cr(s) 2[NO3] (aq) + 2HO(1) +2e=NO4(g) + 4[OH](aq) 2HO(1) + 2e = H(g) + 2[OH]- (aq) E or E" (OH-]=1/V -3.04 -3.03 -2.98 -2.93 -2.87 -2.71 -2.38 -2.37 -2.37 -2.03 -1.66 -1.65 -1.63 -1.56 -1.19 -1.14 -1.13 -1.12 -0.93 -0.92 -0.91 -0,85 -0.82
Step by Step Solution
3.29 Rating (155 Votes )
There are 3 Steps involved in it
To predict qualitatively the outcome of the given experiment at 298 K 25C we need to consider the st... View full answer

Get step-by-step solutions from verified subject matter experts


