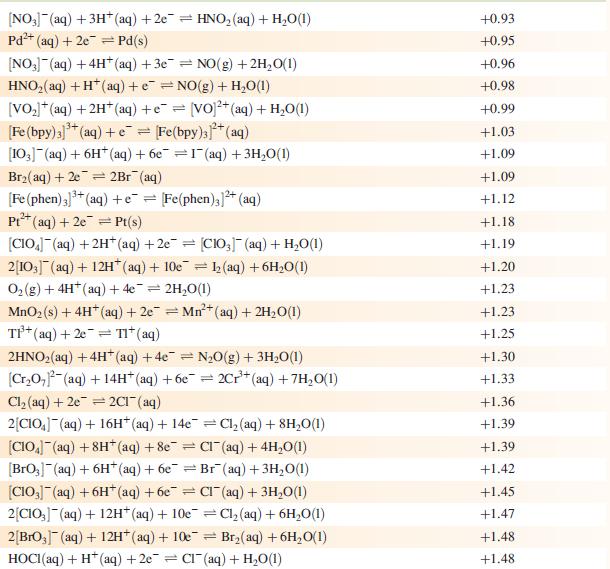
Question: (a) Use appropriate data from Appendix 11 to determine the ratio of the overall stability constants of the complexes [Fe(phen) 3 ] 2+ and [Fe(phen)
(a) Use appropriate data from Appendix 11 to determine the ratio of the overall stability constants of the complexes [Fe(phen)3]2+ and [Fe(phen)3]3+ at 298 K.
(b) Use the data in Fig. 8.2 to construct a Frost–Ebsworth diagram for manganese in aqueous solution at pH 14. Use your diagram to comment on the stability of [MnO4]3− under these conditions.
Figure 8.2
![Acidic solution (pH 0) [MnO4] Alkaline solution (pH 14) [MnO] +0.90 +0.56](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2023/07/64bcdb1675db8_45364bcdb15eef0c.jpg)
Data from Appendix 11
The concentration of each aqueous solution is 1 mol dm−3 and the pressure of a gaseous component is 1 bar (105 Pa). (Changing the standard pressure to 1 atm (101 300 Pa) makes no difference to the values of Eo at this level of accuracy.) Each half-cell listed contains the specified solution species at a concentration of 1 mol dm−3; where the half-cell contains [OH]−, the value of Eo refers to [OH−] = 1 mol dm−3, hence the notation Eo[OH−] = 1
![[HMnO4]- +1.69 [MnO4] +1.51 +2.10 +0.59 +0.27 MnO L +0.95 [MnO4] +0,60](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2023/07/64bcdb17c54f5_45564bcdb174e882.jpg)

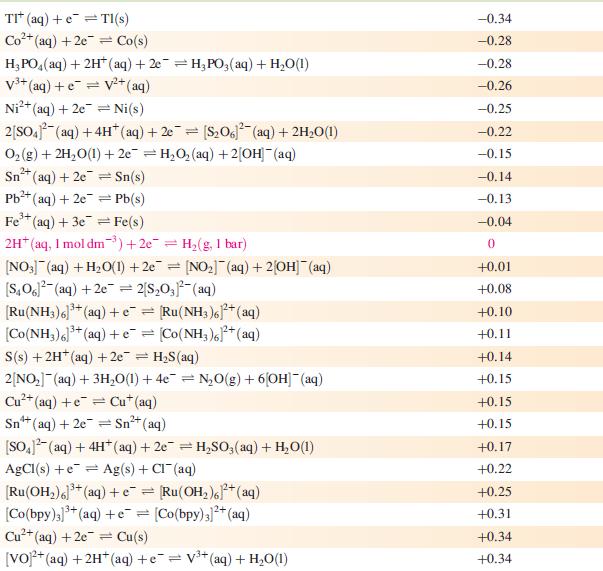
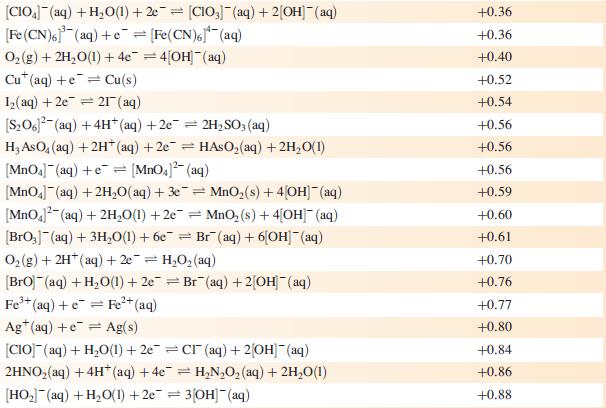

Acidic solution (pH 0) [MnO4] Alkaline solution (pH 14) [MnO] +0.90 +0.56 [HMnO4]- +1.69 [MnO4] +1.51 +2.10 +0.59 +0.27 MnO L +0.95 [MnO4] +0,60 +0.93 Mn+ +1.23 +1.54 MnO Mn+ +0.15 -1.19 -0.04 MnO3 Mn -0.23 Mn(OH) -1.56 Mn
Step by Step Solution
3.46 Rating (159 Votes )
There are 3 Steps involved in it
Part a To determine the ratio of the overall stability constants of the complexes Fephen32 and Fephen3 we can use the provided data from Appendix 11 which includes standard reduction potentials The st... View full answer

Get step-by-step solutions from verified subject matter experts


