Question: Using data from Table 8.1 and from Section 8.3, explain why H 2 is evolved when powdered Ag is heated with a concentrated solution of
Using data from Table 8.1 and from Section 8.3, explain why H2 is evolved when powdered Ag is heated with a concentrated solution of HI.
Data from Table 8.1
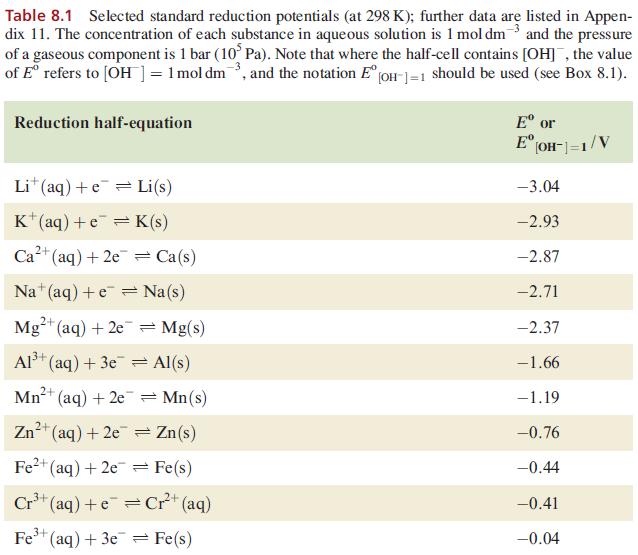
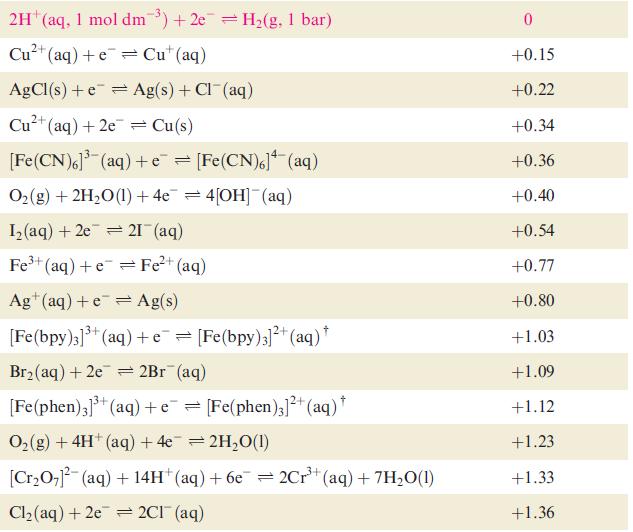
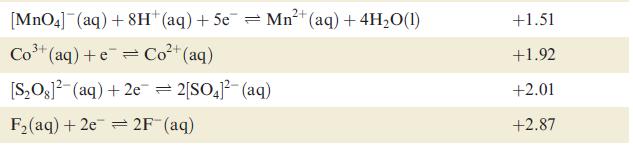
Table 8.1 Selected standard reduction potentials (at 298 K); further data are listed in Appen- dix 11. The concentration of each substance in aqueous solution is 1 mol dm3 and the pressure of a gaseous component is 1 bar (10 Pa). Note that where the half-cell contains [OH], the value of E refers to [OH] = 1 mol dm, and the notation E [OH-]=1 should be used (see Box 8.1). Reduction half-equation Lit (aq) +eLi(s) K+ (aq) + eK(s) 2+ Ca+ (aq) +2e= Ca(s) Na (aq) +eNa(s) 2+ Mg+ (aq) + 2e 3+ Al+ (aq) + 3e 2+ Mn+ (aq) + 2e 1+ (aq) + 2e Fe+ (aq) + 2e 3+ Cr+ (aq) + Zn 3+ Fe+ (aq) + 3e = Mg(s) Al(s) = Mn(s) Zn(s) Fe(s) Cr+ (aq) = Fe(s) E or E [OH-]=1/V -3.04 -2.93 -2.87 -2.71 -2.37 -1.66 -1.19 -0.76 -0.44 -0.41 -0.04
Step by Step Solution
3.44 Rating (176 Votes )
There are 3 Steps involved in it
To understand why H2 is evolved when powdered Ag silver is heated with a concentrated solution of HI hydrogen iodide we need to consider the redox rea... View full answer

Get step-by-step solutions from verified subject matter experts


