Blanchard and Syzdek (1977) formed individual air bubbles in water under nearstatic conditions using a method shown
Question:
Blanchard and Syzdek (1977) formed individual air bubbles in water under nearstatic conditions using a method shown in Fig. P4.9. Air was pushed through an immersed glass capillary to form a slowly growing bubble at its tip. After reaching a critical size and being released, the bubble rose until it rested on an inverted cup attached to a sensitive balance. The balance measured the upward force F that the bubble exerted on the cup. We will denote the inner diameter of the capillary tip and critical bubble diameter as d and D, respectively. Values of D (calculated from F) were obtained as a function of d at 20–22 °C.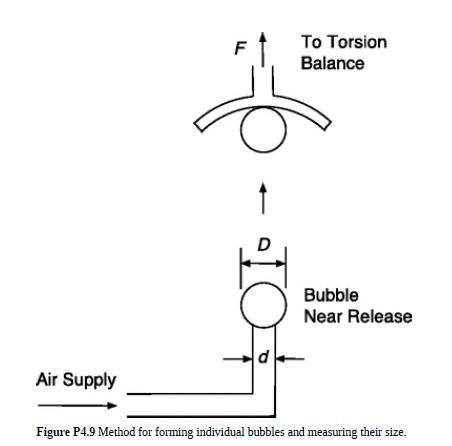
(a) What is the relationship between F and D?
(b) As it nears release from a vertical tube, the actual shape of a bubble will resemble that in Fig. 4.14. The volume V of the “light bulb” equals that of the final sphere of diameter D. Use a force balance on a carefully defined control volume to relate D to d and γ.
(c) Create a log–log plot to compare your predicted curve for D(d) with the experimental results in Table P4.9. How accurate is your expression?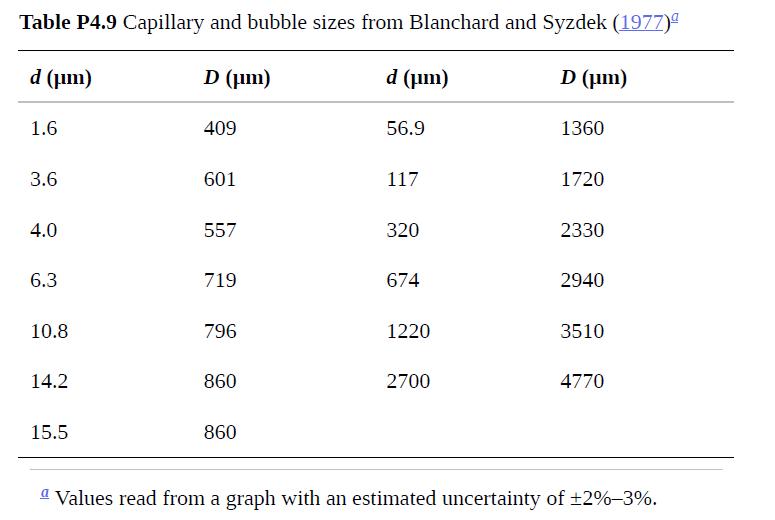
Step by Step Answer:

Introduction To Chemical Engineering Fluid Mechanics
ISBN: 9781107123779
1st Edition
Authors: William M. Deen




