Question: A solution contains 63 different conjugate acid-base pairs. Among them is acrylic acid and acrylate ion, with the equiliborium ratio [acrylate]/[acrylic acid] = 0.75. What
A solution contains 63 different conjugate acid-base pairs. Among them is acrylic acid and acrylate ion, with the equiliborium ratio [acrylate]/[acrylic acid] = 0.75. What is the pH of the solution?
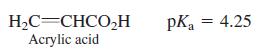
H,C=CHCO,H Acrylic acid pKa = 4.25
Step by Step Solution
3.43 Rating (166 Votes )
There are 3 Steps involved in it
According to the HendersonHassel... View full answer

Get step-by-step solutions from verified subject matter experts


