Dropping-mercury electrodes have been used extensively in analytical chemistry and electrochemical research. As shown in Fig. P8.11,
Question:
Dropping-mercury electrodes have been used extensively in analytical chemistry and electrochemical research. As shown in Fig. P8.11, a small mercury drop of radius a is suspended from a tube in an aqueous electrolyte solution. A constant volume flow rate Q causes the drop to grow until surface tension can no longer support it and it detaches. The time-dependent current caused by an electrochemical reaction at the mercury–water interface is measured up to the moment of detachment. Knowledge of the velocity in the surrounding solution is needed to fully interpret such data.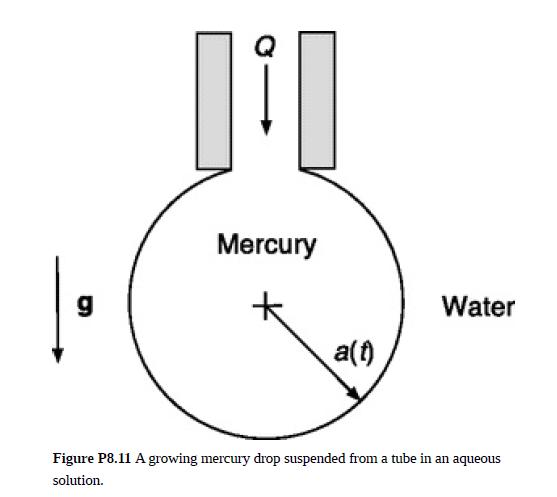
(a) Assuming that Stokes’ equation applies in the electrolyte solution and that vθ and vϕ inside the mercury drop are each negligible, show that the external flow is time-independent and purely radial. Determine vr(r) and ℘(r) in the electrolyte solution.
(b) A reasonable criterion for the use of Stokes’ equation is ![]()
Will Stokes’ equation become less or more accurate as the drop grows? In a typical application, a mercury drop has a radius amax = 0.5 mm just before detachment and a lifetime tmax = 3 s. Over approximately what fraction of that lifetime will Stokes’ equation apply?
Step by Step Answer:

Introduction To Chemical Engineering Fluid Mechanics
ISBN: 9781107123779
1st Edition
Authors: William M. Deen





