Allyl chloride (AC) synthesis provides a simple prototype of many petrochemical processes. 869 kg per hour of
Question:
Allyl chloride (AC) synthesis provides a simple prototype of many petrochemical processes. 869 kg per hour of propylene (C3) are fed with a 1% excess of chlorine (Cl2) at 25°C. The entire process operates at roughly 10 bar. Cl2 is recycled to achieve a 50% excess of Cl2 at the reactor inlet. The reactor conversion is 100% of the propylene to AC and hydrochloric acid (HCl) at 511°C.
The reactor effluent is cooled to 35°C and sent to a distillation column where 98% of the entering AC exits the bottom with 1% of the entering Cl2 and no HCl. This AC product stream exits at 57°C. The tops of the first column are sent to a second column where 99% of the entering Cl2 exits the bottom at 36°C, along with 1% of the entering HCl, all of the AC, and is recycled. The tops of the second column exit at -31°C and are sent for waste treatment. Using the method of Heat of Formation method for the energy balance and ideal gas reference states with Eqn. 2.45 to estimate the heat of vaporization, complete the following.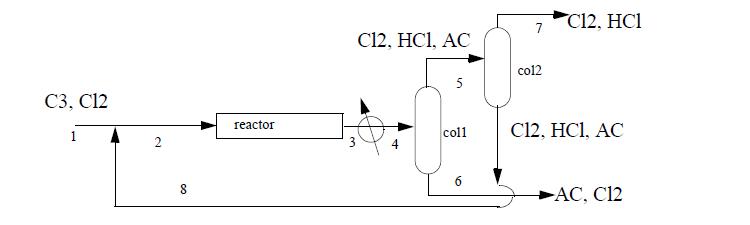
(a) Write a balanced stoichiometric equation for this reaction. (Hint: Check the NIST Web- Book for chemical names and formulas.)
(b) Perform a material balance to determine compositions and flow rates for all streams.
(c) Using only streams (1), (6), (7), calculate the energy balance in MJ/h of the entire process. Does the process involve a net energy need or surplus?
(d) Determine the heat load on the reactor in MJ/h.
(b) Calculate the enthalpies in MJ/h of the feed stream 1.
(c) Calculate the enthalpy in MJ/h of the stream 4 entering the first distillation column.
(d) Calculate the enthalpy in MJ/h of the AC product stream 6.
(e) Calculate the enthalpy in MJ/h of the Cl2 recycle stream 8.
(f) Calculate the enthalpy in MJ/h of the HCl waste stream 7.
Step by Step Answer:

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira





