An A + B mixture exhibits solvation in the liquid phase, which is to be represented using
Question:
An A + B mixture exhibits solvation in the liquid phase, which is to be represented using ideal chemical theory. Because of a Lewis acid/base interaction, the system is expected to form a 1-1 compound.
(a) Which one of the following sets of true mole fractions are correct for the system using an equilibrium constant of 3.2 to represent the complex formation at an apparent composition xA = 0.4?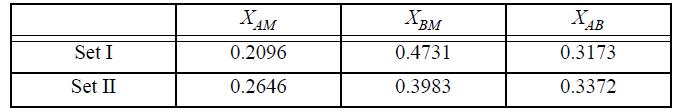
(b) Based on your answer for part (a), what are the apparent activity coefficients of A and B?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:





