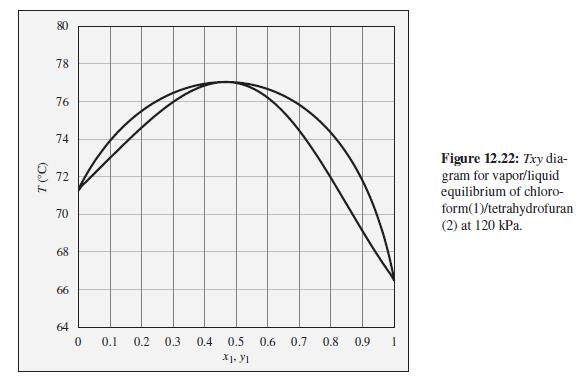
Consider a chloroform(1)/tetrahydrofuran(2) mixture with x 1 = 0.90, initially at 76C and 120 kPa. Describe the
Question:
Consider a chloroform(1)/tetrahydrofuran(2) mixture with x1 = 0.90, initially at 76°C and 120 kPa. Describe the evolution of phases and phase compositions as the temperature is gradually reduced to 66°C.
To the Txy diagram for chloroform(1)/tetrahydrofuran(2) at 120 kPa shown in Fig. 12.22.
Transcribed Image Text:
78 76 74 Figure 12.22: Txy dia- gram for vapor/liquid equilibrium of chloro- form(1)/tetrahydrofuran (2) at 120 kPa. 72 70 68 66 64 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 X1. Y1 T (C)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 44% (9 reviews)
As the temperature of the chloroform1tetrahydrofuran2 mixture is gradually reduced from 76C to 66C i...View the full answer

Answered By

Dulal Roy
As a tutor, I have gained extensive hands-on experience working with students one-on-one and in small group settings. I have developed the ability to effectively assess my students' strengths and weaknesses, and to customize my teaching approach to meet their individual needs.
I am proficient at breaking down complex concepts into simpler, more digestible pieces, and at using a variety of teaching methods (such as visual aids, examples, and interactive exercises) to engage my students and help them understand and retain the material.
I have also gained a lot of experience in providing feedback and guidance to my students, helping them to develop their problem-solving skills and to become more independent learners. Overall, my hands-on experience as a tutor has given me a deep understanding of how to effectively support and encourage students in their learning journey.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:
Students also viewed these Engineering questions
-
Describe the evolution of business-to-business (B2B) e-commerce.
-
Describe the evolution of ERP systems.
-
Describe the evolution of American culinary arts.
-
Greety Food in Ashland, Kentucky, manufactures and markets snack foods. Sita Lee manages the company's fleet of 220 delivery trucks. Lee has been charged with *reengineering* the fleet-management...
-
Whatever can be done by a tariff can be done by a quota. Discuss.
-
The cost of merchandise sold for Kohls Corporation for a recent year was $10,680 million. The balance sheet showed the following current account balances (in millions): Determine the amount of cash...
-
A material processing oven has a first cost of $\$ 16,999$ with a life of 10 years and a salvage value of $\$ 2,500$. The corporate MARR is $12 \%$. If the system provides about $\$ 2,750$ annually,...
-
The College Board provided comparisons of Scholastic Aptitude Test (SAT) scores based on the highest level of education attained by the test takers parents. A research hypothesis was that students...
-
Compute the standard deviation for your two asset portfolio Asset X: Weight = 0.5 Standard deviation = 0.0595 Correlation = 0.3568 Asset Y: Weight = 0.5 Standard deviation = 0.0930 Correlation =...
-
Is reinforcement learning an appropriate abstract model for evolution? What connection exists, if any, between hardwired reward signals and evolutionary fitness?
-
A certain gas is described by the equation of state: Here, b is a constant and is a function of T only. For this gas, determine expressions for the isothermal compressibility and the thermal...
-
Consider a chloroform(1)/tetrahydrofuran(2) mixture with x 1 = 0.10, initially at 80C and 120 kPa. Describe the evolution of phases and phase compositions as the temperature is gradually reduced to...
-
Eastmark Electrical Equipment Manufacturers needs to secure its supply of copper for the next year. The price of copper is extremely volatile because of huge month-to-month variation in demand....
-
Briefly explain the relationship between inferential statistics and sampling.
-
Briefly explain the relationship between sampling cost and sampling error. Give some examples of sampling costs.
-
Briefly explain why we sometimes construct confidence intervals for the population mean.
-
What is an unbiased estimator? What is an efficient estimator? What is a consistent estimator? Why are these concepts important?
-
A quality control engineer knows from past experience that the mean weight for ball bearings is 7.4 oz with a standard deviation of 1.2 oz. Suppose the engineer draws a random sample of 20 ball...
-
Write down all 4 4 permutation matrices that (a) Fix the third row of a 4 4 matrix A; (b) Take the third row to the fourth row: (c) interchange the second and third rows.
-
The Alert Company is a closely held investment-services group that has been very successful over the past five years, consistently providing most members of the top management group with 50% bonuses....
-
A two-stage cascade refrigeration system (see Fig. 93) operates between TC = 210 K and TH = 305 K. Intermediate temperatures are T'C = 255 K and T'H = 260 K. Coefficients of performance of each...
-
A two-stage cascade refrigeration system (see Fig. 93) operates between TC = 210 K and TH = 305 K. Intermediate temperatures are T'C = 255 K and T'H = 260 K. Coefficients of performance of each...
-
The contents of the freezer in a home refrigerator are maintained at - 20C. The kitchen temperature is 20C. If heal leaks amount to 125.000 U per day and if electricity costs $0.08/k Whr, estimate...
-
In the Queries section of the Navigation Pane, right-click Stone Mountain Patients to select it and display the shortcut menu. 3. Click Copy on the shortcut menu. 4. Right-click the empty area near...
-
What is 'audit risk', and discuss/analyze the components of audit risk. b. List the four (4) things involved in the initial audit planning and which should be done early in the audit.
-
K Internal control is a plan of organization and a system of procedures, implemented by company. A. external auditors; management B. external auditors; board of directors OC. internal auditors;...

Study smarter with the SolutionInn App


