Consider a ternary system comprising solute species 1 and a mixed solvent (species 2 and 3). Assume
Question:
Consider a ternary system comprising solute species 1 and a mixed solvent (species 2 and 3). Assume that:
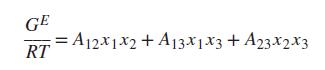
Show that Henry’s constant H1 for species 1 in the mixed solvent is related to Henry’s constants H1,2 and H1,3 for species 1 in the pure solvents by:
![]()
Here x′2 and x′3 are solute-free mole fractions:

Transcribed Image Text:
GE = A12*1x2 + A13x1x3+ A23x2x3 RT
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (6 reviews)
Answer To derive the relationship between Henrys constant H1 for species 1 in the mixed solvent and Henrys constants H12 and H13 for species 1 in the pure solvents we first need to define Henrys law H...View the full answer

Answered By

Aketch Cindy Sunday
I am a certified tutor with over two years of experience tutoring . I have a passion for helping students learn and grow, and I firmly believe that every student has the potential to be successful. I have a wide range of experience working with students of all ages and abilities, and I am confident that I can help students succeed in school.
I have experience working with students who have a wide range of abilities. I have also worked with gifted and talented students, and I am familiar with a variety of enrichment and acceleration strategies.
I am a patient and supportive tutor who is dedicated to helping my students reach their full potential. Thank you for your time and consideration.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:
Students also viewed these Engineering questions
-
The following information is related to the Lilliput Veterinary Clinic for April 2010, the firms first month of operation: Veterinarian salaries for April ............... $16,200 Assistants salaries...
-
The following information is related to North Zulch Veterinary Clinic for April 2013, the firms first month of operation: Veterinarian salaries for April ............... $ 8,100 Assistants salaries...
-
The following information is related to manufacturing office furniture at Outreach, Inc.: a. Accept and arrange raw materials in inventory1 day. b. Store raw materials in inventory5 days. c. Issue...
-
1. Is religious training mandatory at all of the schools in question? 2. Does the Court see inevitable church-state entanglements if the Board were allowed to exercise jurisdiction over teachers in...
-
It is usually asserted that public utilities such as electric companies and gas companies are natural monopolies, but an assertion is not proof. How would you go about trying to prove (disprove) that...
-
Sullivan sold t-shirts with the name Boston Marathon and the year of the race imprinted on them. The Boston Athletic Association (BAA) sponsors and administers the Boston Marathon and has used the...
-
Explain synchronous and asynchronous communication in relation to elearning.
-
Myles Etter and Crystal Santori are partners who share in the income equally and have capital balances of $210,000 and $62,500, respectively. Etter, with the consent of Santori, sells one-third of...
-
Identify and describe two network management and security process controls that can be put in place to ensure network security. a.Describe network management (system) including i.Network Management...
-
A cone floats in glycerin (SG = 1.26), as shown in the figure. Find the mass of the cone. In the figure, h= 30 cm. The radius of the cone Ris 20 cm. SG = 1.26 60 cm The mass of the cone is kg.
-
With V 2 = V 2 , Eq. (15.66) for the osmotic pressure may be represented as a power series in x 1 : Reminiscent of Eqs. (3.33) and (3.34), this series is called an osmotic virial expansion. Show that...
-
It is possible in principle for a binary liquid system to show more than one region of LLE for a particular temperature. For example, the solubility diagram might have two side-by-side islands of...
-
(a) If 0 < c < 1, show that 0 < c2 < c < 1. (b) If 1 < c, show that 1 < c < c2.
-
Ross (1995) puts forward that there are three sources of value in a typical capital budgeting project. Explain these three sources using examples.
-
Given that the Venezuela Caracas Stock Exchange was up by over 80 per cent for 2011, why didnt all investors put their money in Venezuela?
-
What is meant by the term return? What is the difference between monetary returns and percentage returns? Do monetary or percentage returns matter more to investors? Provide an example to explain...
-
What is data mining? Is there anything wrong with measuring the performance of an Irish growth fund portfolio manager against a benchmark composed of Greek equities?
-
What do we mean by risk? In long-term investments, equities tend to give higher returns than bonds. Why then, do all investors not invest in equities? Are such investors irrational?
-
In each part, find the maximum and minimum values of the quadratic form subject to the constraint x21 + x22 = 1, and determine the values of x1 and x2 at which the maximum and minimum occur. 7x21 +...
-
Use the formula to determine the value of the indicated variable for the values given. Use a calculator when one is needed. When necessary, use the key on your calculator and round answers to the...
-
The gas condensate from a new gas well in Prudhoe Bay, Alaska has the following weight% of C5, C10, C15, C20, C25, C30, C35, C40, C45, C50, and >C50, respectively: 1, 4, 7, 10, 12, 12, 12, 12, 8, 8,...
-
Determine the solubility curve for naphthalene in the specified solvent, and compare with the literature data: (a) Acetic acid 9 (b) n-hexane 9 (c) Cyclohexanol 10 (d) Acetone 9 (e) Chloroform 11 (f)...
-
Determine the ideal solubility of phenanthrene in any solvent at 20C. Then predict the solubility and compare with the experimental solubility (shown in parentheses) for the specified solvent and...
-
You invest 50% of your financial assets in the Vanguard Total Stock Market ETF (VTI) and 50% in the Vanguard Total Bond Market ETF (BND). VTI has an expected return of 8% and a standard deviation of...
-
You have found the car you would like to purchase. The negotiated price = $57,500 and you are taking out a loan with a 7.5% APR compounded monthly for 6 years. What are your monthly payments? Car...
-
Suppose a male member currently aged 35, entered service at his age of 25, has a current salary of $75, 000 and total past salary of $650, 000. Assume that salaries increase at the beginning of each...

Study smarter with the SolutionInn App


