For the reversible isothermal compression of a liquid for which and may be assumed independent
Question:
For the reversible isothermal compression of a liquid for which β and κ may be assumed independent of pressure, show that: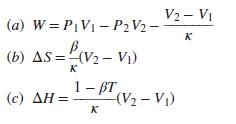
Do not assume that V is constant at an average value, but use Eq. (3.6) for its P dependence (with V2 replaced by V). Apply these equations to the conditions stated in Prob. 6.9. What do the results suggest with respect to use of an average value for V?
Eq. (3.6)
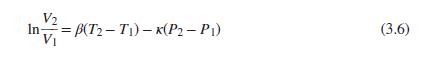
Problem 6.9
One kilogram of water (V1 = 1003 cm3·kg−1) in a piston/cylinder device at 25°C and 1 bar is compressed in a mechanically reversible, isothermal process to 1500 bar. Determine Q, W, ΔU, ΔH, and ΔS given that β = 250 × 10−6 K−1 and κ = 45 × 10−6 bar−1. A satisfactory assumption is that V is constant at its arithmetic average value.
Step by Step Answer:

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart





