For the same mixture and experimental conditions as problem 15.4, calculate the fugacity of each component in
Question:
For the same mixture and experimental conditions as problem 15.4, calculate the fugacity of each component in the mixture, f(^)i. Use methods (a) - (e).
Data from problem 15.4:
Calculate the molar volume of a binary mixture containing 30 mol% nitrogen(1) and 70 mol% n-butane(2) at 188°C and 6.9 MPa by the following methods.
(a) Assume the mixture to be an ideal gas.
(b) Assume the mixture to be an ideal solution with the volumes of the pure gases given by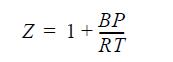
and the virial coefficients given below.
(c) Use second virial coefficients predicted by the generalized correlation for B.
(d) Use the following values for the second virial coefficients.
Data:![]()
(e) Use the Peng-Robinson equation.
Step by Step Answer:

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira





