For their homework assignment three students, Julie, John, and Jacob, were working on the formation of ammonia.
Question:
For their homework assignment three students, Julie, John, and Jacob, were working on the formation of ammonia. The feed is a stoichiometric ratio of nitrogen and hydrogen at a particular T and P.
Julie, who thought in round numbers of product, wrote: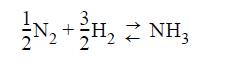
John, who thought in round numbers of nitrogen, wrote:![]()
Jacob, who thought in round numbers of hydrogen, wrote: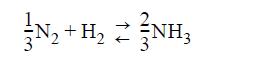
(a) How will John’s and Jacob’s standard state Gibbs energy of reactions compare to Julie’s?
(b) How will John’s and Jacob’s equilibrium constants be related to Julie’s?
(c) How will John’s and Jacob’s equilibrium compositions be related to Julie’s?
(d) How will John’s and Jacob’s reaction coordinate values be related to Julie’s?
Step by Step Answer:

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira





