One method for the manufacture of synthesis gas depends on the vapor phase catalytic reaction of methane
Question:
One method for the manufacture of synthesis gas depends on the vapor phase catalytic reaction of methane with steam according to the equation CH4 + H2O ⇆ CO + H2. The only other reaction which ordinarily occurs to an appreciable extent is the water-gas shift reaction. Gibbs energies and enthalpies for the problem are tabulated below in kJ/mol.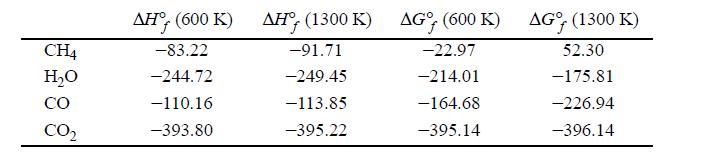
Compute the equilibrium compositions based on a 1:1 feed ratio at 600 K and 1300 K and 1 bar and 100 bars.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:





