Starting with Eq. (6.9), show that isotherms in the vapor region of a Mollier (HS) diagram have
Question:
Starting with Eq. (6.9), show that isotherms in the vapor region of a Mollier (HS) diagram have slopes and curvatures given by: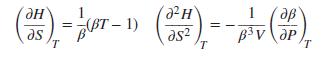 Here, β is volume expansivity. If the vapor is described by the two-term virial equation in P, Eq. (3.36), what can be said about the signs of these derivatives? Assume that, for normal temperatures, B is negative and dB/dT is positive.
Here, β is volume expansivity. If the vapor is described by the two-term virial equation in P, Eq. (3.36), what can be said about the signs of these derivatives? Assume that, for normal temperatures, B is negative and dB/dT is positive.
Eq. (6.9)
![]()
Eq. (3.36)

Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:





