Suppose a vessel contains an equimolar mixture of chloroform(1) and triethylamine(2) at 25C. The following data are
Question:
Suppose a vessel contains an equimolar mixture of chloroform(1) and triethylamine(2) at 25°C. The following data are available at 25°C: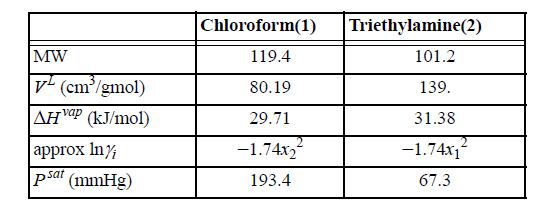
(a) If the pressure in the vessel is 90 mmHg, is the mixture a liquid, a vapor, or both liquid and vapor? Justify your answer.
(b) Provide your best estimate of the volume of the vessel under these conditions. State your assumptions.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:





