Ten thousand (10,000) kgh 1 of an 80-wt-% H 2 SO 4 solution in water at 300
Question:
Ten thousand (10,000) kg·h−1 of an 80-wt-% H2SO4 solution in water at 300 K is continuously diluted with chilled water at 280 K to yield a stream containing 50-wt-% H2SO4 at 330 K.
(a) What is the mass flow rate of chilled water in kg·h−1?
(b) What is the rate of heat transfer in kJ·h−1 for the mixing process? Is heat added or removed?
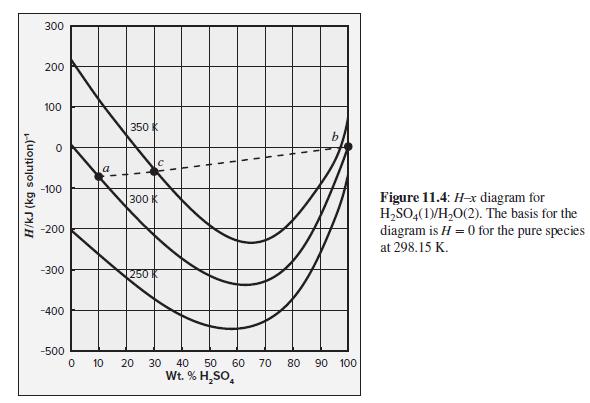
(c) If the mixing occurred adiabatically, what would be the temperature of the product stream? Assume here the same inlet conditions and the same product composition as for part (b). Heat of solution data is available in Fig. 11.4.
Fig. 11.4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:





