The hydrogen halides are unusual. For example, here are the critical properties of various hydrogen halides: Experimental
Question:
The hydrogen halides are unusual. For example, here are the critical properties of various hydrogen halides: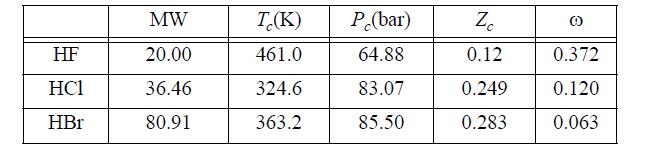
Experimental data for the vapor pressure and the apparent molecular weight of HF vapor are as follows: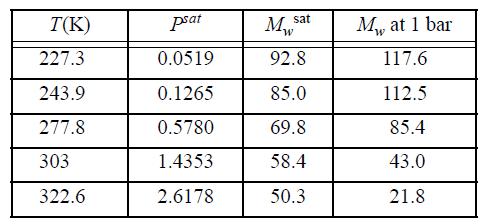
These apparent molecular weights have been found by measuring the mass density of the vapor and comparing with an ideal gas of molecular weight 20. Assuming that HF forms only monomers and hexamers, use the ESD EOS with c = q = 1 for both monomer and hexamer to match this value of Zc, and fit the vapor density data as accurately as possible in the least squares sense.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:





