Question: Using the van Laar model and the data from problem 11.3, estimate the total pressure and composition of the vapor in equilibrium with a 20
Using the van Laar model and the data from problem 11.3, estimate the total pressure and composition of the vapor in equilibrium with a 20 mol% ethanol(1) solution in water(2) at 78.15°C.
Data from problem 11.3
After fitting the two-parameter Margules equation to the data below, generate a P-x-y diagram at 78.15 °C.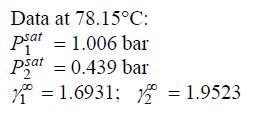
Data at 78.15C: psat = 1.006 bar 1 psat = 0.439 bar = 1.6931; 2 = 1.9523
Step by Step Solution
3.39 Rating (158 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


