What is the composition of the vapor phase in equilibrium with a liquid phase ethanol(1)/ethyl acetate(2) mixture
Question:
What is the composition of the vapor phase in equilibrium with a liquid phase ethanol(1)/ethyl acetate(2) mixture of the following compositions at P = 1 bar?
(a) x1 = 0.1
(b) x1 = 0.2
(c) x1 = 0.3
(d) x1 = 0.45
(e) x1 = 0.6
(f) x1 = 0.8
(g) x1 = 0.9
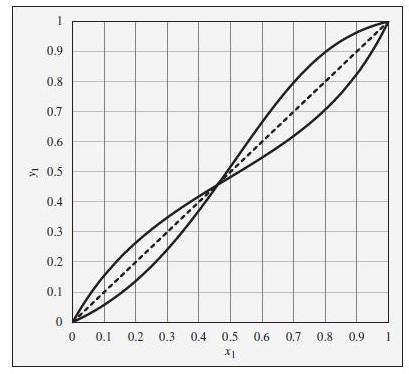
To the xy diagram provided in Fig. 12.23. This diagram shows xy curves both for ethanol(1)/ethyl acetate(2) and for chloroform(1)/tetrahydrofuran(2), both at a constant pressure of 1 bar. The curves are intentionally unlabeled.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:





