When only one component of a binary mixture is volatile, the pressure over the mixture is determined
Question:
When only one component of a binary mixture is volatile, the pressure over the mixture is determined entirely by the volatile component. The activity coefficient for the volatile component can be determined using modified Raoult’s law and an activity coefficient model can be fitted. The model will satisfy the Gibbs-Duhem equation and thus an activity coefficient prediction can be made for the nonvolatile component. Consider a solution of sucrose and water. The sucrose is nonvolatile. The bubble pressures of water (1) + sucrose (2) solutions are tabulated below at three temperatures.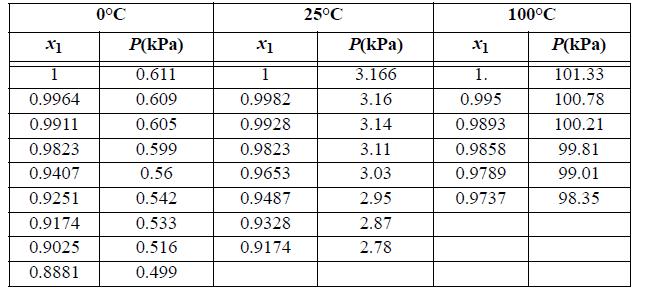
(a) Fit the one-parameter Margules equation to the water data at the temperature(s) specified by your instructor. Report the values of A12.
(b) Prepare a table of γ1 values and plot of the experimental and fitted/predicted versus x1 for water and sucrose over the range of experimental compositions for the temperature(s) specified by your instructor.
(c) Prepare a table of values and on the same plot as (b) add a curve for lnγ2* for the temperature(s) specified by your instructor.
(d) Prepare a table of values and a plot of osmotic pressure (in MPa) for the solution versus C2 (g/L) at 25°C. The density at 25°C can be estimated using ρ(g/mL) = 0.99721 + 0.3725w2 + 0.16638w2 2 where w2 is wt. fraction sucrose. Include a curve of the osmotic pressure expected for an ideal solution.
(e) Calculate the osmotic pressure (MPa) using the activity of water modeled with the oneparameter Margules equation at 25°C fitted in part (a). Add it to the plot in part (d).
(f) Calculate the second and third osmotic virial coefficients (for concentration units of g/L) at 25°C by fitting the calculations from part (d). Add the modeled osmotic pressure to the plot from (d).
(g) From the temperature dependence of the one-parameter Margules parameter fitted in (a), show that the parameter may be represented with f(1/T(K)). Then provide a model for the excess enthalpy and the parameter value(s) that represent the experimental data.
Step by Step Answer:

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira




