Write and balance the chemical reaction of carbon monoxide forming solid carbon and carbon dioxide vapor. Determine
Question:
Write and balance the chemical reaction of carbon monoxide forming solid carbon and carbon dioxide vapor. Determine the equilibrium constant at 700 K and 750 K. Will solid carbon form at the conditions of problem 17.14?
Data from problem 17.14
Hydrogen gas can be produced by the following reactions between propane and steam in the presence of a nickel catalyst: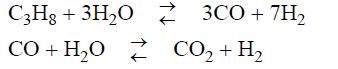
Neglecting any other competing reactions:
(a) Compute the equilibrium constants at 700 K and 750 K.
(b) What is the equilibrium composition of the product gas if the inlet to the catalytic reactor is propane and steam in a 1:5 molar ratio at each of the temperatures and 1 bar?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:





