Imagine a hydrogen atom at the center of an infinite spherical well of radius b. We will
Question:
Imagine a hydrogen atom at the center of an infinite spherical well of radius b. We will take b to be much greater than the Bohr radius (α) , so the low-n states are not much affected by the distant “wall” at r = b . But since u (b) = 0 we can use the method of Problem 2.61 to solve the radial equation (4.53) numerically.
(a) Show that vj (in Problem 2.61) takes the form
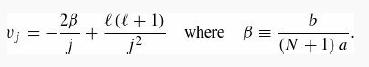 (b) We want Δr << a (so as to sample a reasonable number of points within the potential) and a << b (so the wall doesn’t distort the atom too much). Thus
(b) We want Δr << a (so as to sample a reasonable number of points within the potential) and a << b (so the wall doesn’t distort the atom too much). Thus
![]() Let’s use β = 1/50 and N = 1000. Find the three lowest eigenvalues of H, for ℓ = 0, ℓ = 1, and ℓ = 2, and plot the corresponding eigenfunctions. Compare the known (Bohr) energies (Equation 4.70).
Let’s use β = 1/50 and N = 1000. Find the three lowest eigenvalues of H, for ℓ = 0, ℓ = 1, and ℓ = 2, and plot the corresponding eigenfunctions. Compare the known (Bohr) energies (Equation 4.70).
Equation 4.53
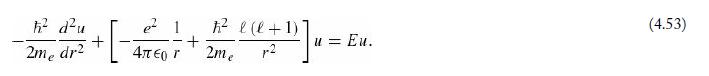
Equation 4.70

Step by Step Answer:

Introduction To Quantum Mechanics
ISBN: 9781107189638
3rd Edition
Authors: David J. Griffiths, Darrell F. Schroeter





