3 moles of pure water are adiabatically mixed with 1 mole of pure ethanol at a constant...
Question:
3 moles of pure water are adiabatically mixed with 1 mole of pure ethanol at a constant pressure of 1 bar. The initial temperatures are the pure components are equal. If the fi nal temperature is measured to be 311.5 K, determine the initial temperature. The enthalpy of mixing between water (1) and ethanol (2) has been reported to be fi t by: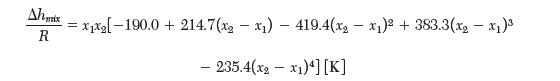
Transcribed Image Text:
Ahmix R =x[-190.0+ = 214.7(x-x) 419.4(x-x) + 383.3(x - x) - 235.4(x-x1)4] [K]
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
A stream of pure water fl owing at 1 mol/s is adiabatically mixed with a stream containing equamolar amounts of water and ethanol, also fl owing at 1 mol/s. This steady-state process occurs at a...
-
Mays and McCovey are beer-brewing companies that operate in a duopoly (two-firm oligopoly). The daily marginal cost (MC) of producing a can of beer is constant and equals $0.80 per can. Assume that...
-
a. Example 13-1: Batch Reactor with an Exothermic Reaction Wolfram 1. Adiabatic Case: Use Wolfram to see whether you can find a trajectory that is ready to ignite and whose trajectory looks like a...
-
Calculate the binding energy per nucleon for a 14/7N nucleus.
-
Using data from the U.S. Census Bureau and other sources, www.nerdwallet.com estimated that considering only the households with credit card debts, the average credit card debt for U.S. households...
-
Susan and Tom have annual fixed and variable expenses of \($100,000.\) They each earn \($80,000\) for a combined earned income of \($160,000.\) Susan has a small trust fund which provides minimal...
-
How does globalization both support and threaten the nationstate?
-
Owner Shan Lo is considering franchising her Noodles by Lo restaurant concept. She believes people will pay $6.50 for a large bowl of noodles. Variable costs are $3.25 per bowl. Lo estimates monthly...
-
On January 1, 2024, Bloomfield Enterprises purchases a building for $327,000, paying $57,000 down and borrowing the remaining $270,000, signing a 7%, 10-year mortgage. Installment payments of...
-
Brian and Corrine Lee are married taxpayers filing jointly. They live in the home they own, located at 3301 Pacific Coast Hwy., Laguna Beach, CA 92651. Brian is an optometrist who owns his business;...
-
Fit the data in Problem 6.53 to the form: (a) Use the analytical method to come up with an expression of the partial molar volume of ethanol. (b) Determine the values at x1 = 0.8, x1 = 0.4, and x1 =...
-
Repeat Example 5.4 using the LeeKesler generalized correlation data for enthalpy departure to account for nonideal behavior.
-
In the application of the equity method, how should dividends from the investee be accounted for? Why?
-
What must a prospective purchaser of land do to ensure that the vendor has the right to transfer the property clear of any claims? Does the purchase of title insurance change this answer?
-
In what important respect has the British Columbia Frustrated Contracts Act provided a fairer solution when a contract is frustrated?
-
What is the purpose of a survey?
-
Define tenancy at will, term certain, overholding tenant, subletting, eviction, forfeiture, surrender , and quiet enjoyment .
-
A firm of public accountants has for years been auditing the accounts of a charitable organization without charge. It now appears that the treasurer of the organization has absconded with a sizeable...
-
Valentine Corporation was organized on June 30, 2008. After 2 1/2 years of profitable operations, the Equity section of Valentines balance sheet was as follows: Contributed capital: Common stock , $5...
-
What are three disadvantages of using the direct write-off method?
-
How would the energy versus dihedral angle plot for 2-methylpropane (isobutene) differs from that for propane?
-
Draw an energy versus dihedral angle plot for the conformations of 2, 3-dirnethylbutane about the C-2C-3 bond.
-
Discuss the geometry and the types of strain present in these compounds: (a) Cyclopropane (b) Cyclobutene (c) Cyclopentane (d) Cyclohexane (e) Cyclodecane
-
The practice you are about to join as a new FNP will become part of a statewide telehealth system to link community-based clinics throughout the state. Access to specialty care has become very...
-
The grandfather of all text-based adventure games is Colossal Cave Adventure, which also went by the names Adventure and ADVENT (on early systems with file name limits). Adventure was created by Will...
-
Mei is working from home and speaking with her department manager on a Voice over IP (VoIP) phone connection. This technology allows telephone conversations to be routed over the Internet. During a...

Study smarter with the SolutionInn App


