Consider a binary mixture of a and b at T = 300 K and P = 40
Question:
Consider a binary mixture of a and b at T = 300 K and P = 40 kPa. A graph of the fugacity of species a as a function of mole fraction is shown below. Use Henry’s law as the reference state for species a and the Lewis/Randall rule for species b. Show all your work.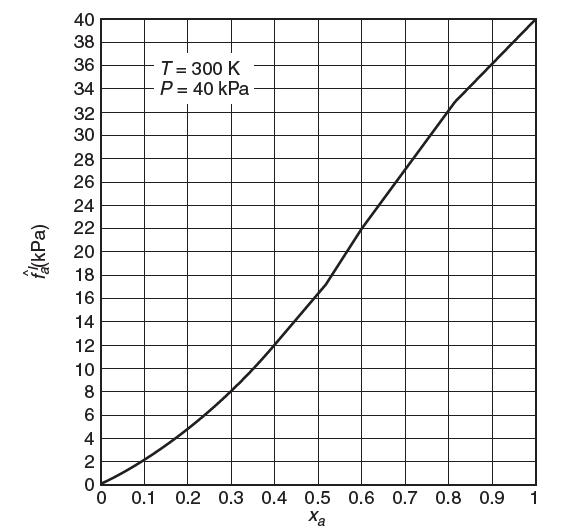
(a) What is the Henry’s law constant, Ha, for species a?
(b) What is the activity coeffi cient for species a at xa = 0.4? At xa = 0.8? (remember the Henry’s law reference state!). Show your work.
(c) Is the activity coeffi cient for species b at xa = 0.4 greater than or less than 1? Explain.
(d) Is the a-b interaction stronger than the pure species interactions? Explain.
(e) Consider the vapor phase to be ideal. What is the vapor-phase mole fraction of a in equilibrium with 40% liquid a?
Step by Step Answer:





