Question: Example 8.6 illustrates how you solve a dew-point calculation for a binary mixture of a nonideal liquid and a nonideal gas with T known. This
Example 8.6 illustrates how you solve a dew-point calculation for a binary mixture of a nonideal liquid and a nonideal gas with T known. This problem corresponds to quadrant I in Figure 8.2.
Develop an analogous solution for the bubble point with the liquid-phase mole fractions and T known (quadrant II). As in Example 8.6, use the van der Waals equation for vapor nonideality and the three-suffi x Margules equation for liquids. Assume that critical properties, liquid volumes, and Antoine coeffi cients for each species are readily available and that the three-suffi x Margules parameters have been determined.
Example 8.6
At high pressures, both the vapor and liquid phases may be nonideal. Consider a binary mixture of a and b with vapor-phase mole fraction and T known. Develop a set of equations and a solution algorithm to determine the composition in the liquid phase and the system pressure.
Use the van der Waals equation to quantify deviations from ideality in the vapor and the threesuffi x Margules equation to model the nonideal liquid. Assume that critical properties, liquid volumes, and Antoine coeffi cients for each species are readily available and that the threesuffi x Margules parameters have been determined.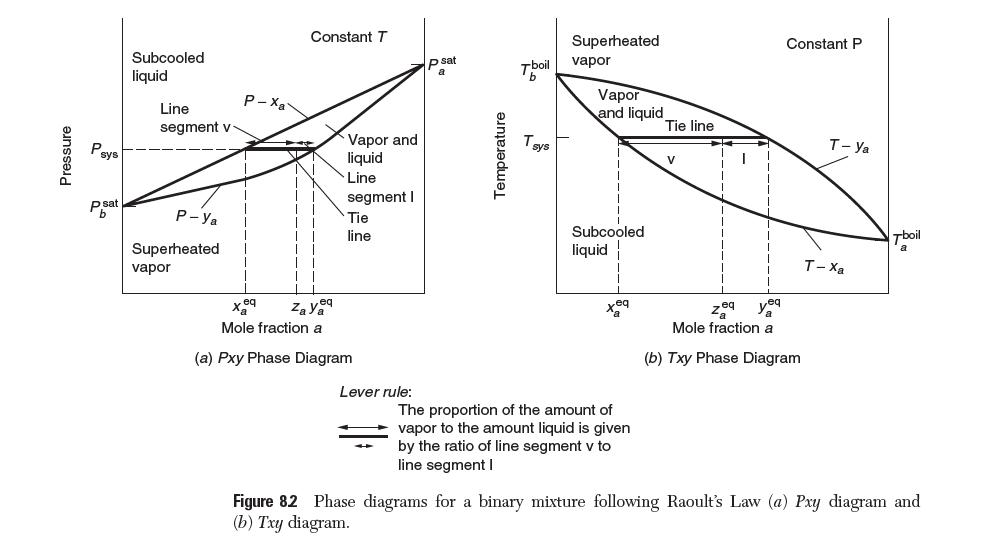
Pressure Psys p sat b Subcooled liquid Line segment v P-Ya Superheated vapor P-Xa eq Constant T eq Vapor and liquid Line segment I Tie line Zaya Mole fraction a (a) Pxy Phase Diagram Lever rule: p sat a Temperature Tboil T sys Superheated vapor Vapor and liquid Subcooled liquid The proportion of the amount of vapor to the amount liquid is given by the ratio of line segment v to line segment I Tie line V 1 Constant P Zeq yea Mole fraction a (b) Txy Phase Diagram T-Ya T-Xa Tboil a Figure 82 Phase diagrams for a binary mixture following Raoult's Law (a) Pxy diagram and (b) Txy diagram.
Step by Step Solution
3.34 Rating (169 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


