Consider a mixture of 1-propanol (a) and water (b) in vapor-liquid equilibrium at 25C. The liquid has
Question:
Consider a mixture of 1-propanol
(a) and water
(b) in vapor-liquid equilibrium at 25°C. The liquid has a mole fraction xa = 0.2. The three-suffi x Margules equation parameters are: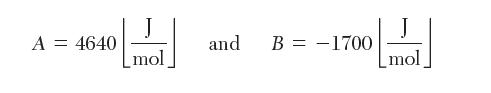
In addition, propylene
(c) can form via chemical reaction in the gas phase. To simplify the calculations you may assume that no propylene exists in the liquid (i.e., xc = 0).
(a) Write the chemical reaction that occurs in the vapor, and calculate the numerical value of the equilibrium constant.
(b) If the gas phase reaction proceeds to equilibrium, calculate the system pressure. You will need to account for both phase equilibrium and chemical reaction equilibrium.
(c) Calculate the mole fractions of a, b, and c in the vapor.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





