Question: Liquidvapor equilibrium data have been collected for a binary system of benzene (1)cyclohexane (2) at 60C. Mole fraction of liquid and vapor vs. total pressure
Liquid–vapor equilibrium data have been collected for a binary system of benzene (1)–cyclohexane (2) at 60°C. Mole fraction of liquid and vapor vs. total pressure are reported in the table below.
From these data, determine the value of the two-suffi x Margules parameter, A. Compare your result to that obtained in Example 7.13. What value do you think is more accurate?
Example 7.13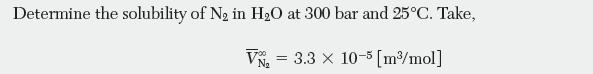
X1 0 0.0672 0.2261 0.3201 0.432 0.5203 0.6029 0.7095 0.7952 0.8752 0.8932 1 Y 0 0.0912 0.267 0.3526 0.448 0.5203 0.5895 0.677 0.7563 0.8386 0.86 1 P [Pa] 51,857 53,431 55,939 56,741 57,527 57,633 57,432 56,989 56,095 54,934 54,629 52,190
Step by Step Solution
3.44 Rating (157 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


