Question: Repeat Problem 2.34, except consider now the combustion products contain CO (g) as well as CO2 (g) and H2O (g) with the ratio of CO2/CO
Repeat Problem 2.34, except consider now the combustion products contain CO (g) as well as CO2 (g) and H2O (g) with the ratio of CO2/CO produced being 4/1. All other conditions remain the same as in Problem 2.34.
Problem 2.34
One kg of liquid n-octane (C8H18) is placed in a closed rigid container with 50% excess air at 100°C and 1 bar. It undergoes an isothermal process in which it reacts and completely combusts to form CO2 (g) and H2O (g). State any assumptions that you make in solving this problem. Take the heat capacity of liquid n-octane to be: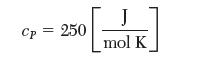
Cp = 250 J mol K
Step by Step Solution
★★★★★
3.51 Rating (158 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


