One kg of liquid n-octane (C8H18) is placed in a closed rigid container with 50% excess air
Question:
One kg of liquid n-octane (C8H18) is placed in a closed rigid container with 50% excess air at 100°C and 1 bar. It undergoes an isothermal process in which it reacts and completely combusts to form CO2 (g) and H2O (g). State any assumptions that you make in solving this problem. Take the heat capacity of liquid n-octane to be: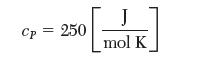
(a) What is the fi nal system pressure?
(b) How much heat must be removed to keep the chamber at 100°C?
(c) Consider the same amount of C8H18 reacts with a stoichiometric amount of O2 and forms the same products, how do you think the value you calculated in part B will change? (More Q, Less Q, No change in Q). Conceptually explain your answer. No calculatio
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





