The reaction of vapor components A and B to form desired solid product C, vapor byproducts D
Question:
The reaction of vapor components A and B to form desired solid product C, vapor byproducts D and F, and solid by-product E is described by the following three reactions: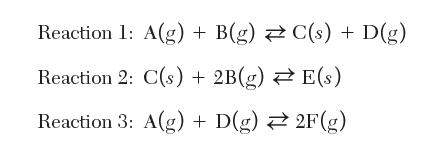
At a temperature of 500 K and a pressure of 50 kPa, and a feed ratio of 1 mol A to 3 mol B, the equilibrium extents of reaction are: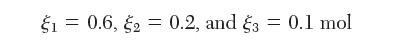
(a) Calculate the values of the equilibrium constants K1, K2, and K3.
(b) How would you change the system pressure to increase the yield of C(s) relative to E(s)?
Explain.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





