Your supervisor has assigned you to obtain parameters A and B for the three-suffi x Margules equation
Question:
Your supervisor has assigned you to obtain parameters A and B for the three-suffi x Margules equation to input in the company’s phase equilibrium computer database. The binary mixture of interest is benzene
(a) in 1-propanol
(b) at 75°C. You cannot fi nd any values in the literature for the three-suffi x Margules equation, but you do fi nd the following values from the van Laar (vL) equation: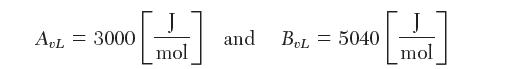
(a) Use these values to estimate the three-suffi x Margules equation parameters A and B.
(b) Calculate the fugacity of benzene in the liquid,![]() in a mixture of 30 mole% benzene at 75°C and 81 kPa.
in a mixture of 30 mole% benzene at 75°C and 81 kPa.
(c) If this liquid mixture is in equilibrium with its vapor, what is the mole fraction of benzene in the vapor?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





