A solution is prepared by dissolving 5.00 g of caffeine in 100.0 g of carbon tetrachloride. The
Question:
A solution is prepared by dissolving 5.00 g of caffeine in 100.0 g of carbon tetrachloride. The solution is cooled and the temperature plotted over time:
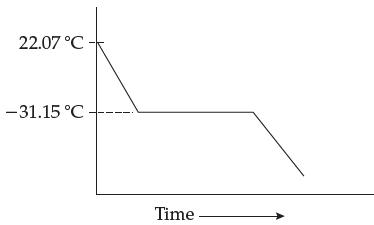
(a) What is the molar mass of caffeine?
(b) Combustion analysis reveals that the empirical formula of caffeine is C4H5N2O. What is the molecular formula?
Transcribed Image Text:
22.07 °C -31.15 °C Time
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
To determine the molar mass of caffeine from the given information well utilize the freezing point d...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
A solution is prepared by dissolving 396 g of sucrose (C12H22O11) in 624 g of water. What is the vapor pressure of this solution at 30C? (The vapor pressure of water is 31.8 mmHg at 30C.)
-
A solution is prepared by dissolving table salt, sodium chloride, in water at room temperature. a. Assuming there is no significant change in the volume of water during the preparation of the...
-
Propionic acid has one acidic proton per molecule. A solution is prepared by dissolving 0.273 g of propionic acid in enough water to yield 100.0 mL of solution. This solution is neutralized by 36.82...
-
Sodium nitrite (NaNO2) reacted with 2-iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula C8H17NO2 in a combined yield of 88%. Suggest reasonable structures...
-
The truck purchased in Exercise 18-2A is expected to be used for 96,000 miles over its eight-year useful life. Using the units-of production method, calculate the depreciation expense for the first...
-
The new director of special events at a large university has decided to completely revamp graduation ceremonies. Toward that end, a PERT chart of the major activities has been developed. The chart...
-
Plaintiffs W. O. and J. C. Lucy had wanted to purchase Ferguson Farm from the Zehmers for at least eight years. One night, Lucy stopped by the establishment the Zehmers operated and said that he bet...
-
1. Should ICANNs actions be judged under the rule of reason or be deemed a per se violation of Section 1 of the Sherman Act? 2. Should ICANNs action be viewed as a horizontal or a vertical restraint...
-
Assume the company is transitioning from a traditional file environment to a database management system / relational database. - Describe and explain the challenges / issues in transitioning from a...
-
What are (a) The boiling point (b) The freezing point of a solution made by dissolving 5.75 g of solid cetyl alcohol, C 16 H 3 4 O, in 100.0 mL of benzene? (The density of benzene is 0.874 g/mL.)
-
What are (a) The boiling point (b) The freezing point of a solution made by dissolving 38.60 g of sodium phosphate in 200.0 mL of water? (The density of water is 1.000 g/mL.)
-
Validity of measures is a critical concept in hiring: it is needed to identify those who will be better workers, and it is needed to legally defend the selection process. In the case of using social...
-
When the Federal Reserve Bank is aggressively buying huge amounts of U.S. government bonds, offering great low-cost deals on Repos to all U.S. banks, and if they are increasing the amount of...
-
Consider a force of 5N[NW], then a force of 5N [SW]. Determine the equilibrant of the resultant of the forces? Draw a diagram showing the resultant and the equilibrant. Show your work
-
A Field Poll Survey reported that 66% of registered voters in a state approved of allowing two people of the same gender to marry and have regular marriage laws apply to them. Among 18 to 39 year...
-
National Shops, Incorporated reported the following amounts on its balance sheet as of December 31, 2022: Inventory $ 325,000 Notes payable 100,000 Cash 150,000 Common stock 750,000 Net property,...
-
What fundamental components characterize team dynamics, and how do these dynamics substantively influence the collective efficacy and productivity of the team?
-
Runzheimer International publishes data on overseas business travel costs. They report that the average per diem total for a business traveler in Paris, France, is $349. Suppose the shape of the...
-
Catherine (aged 42) and Johnson (aged 45) have been married for 12 years. Johnson is a project manager of an event company at a monthly salary of $55,000 with an additional one-month salary of...
-
The state of stress at a point is shown on the element. Determine (a) the principal stresses and (b) the maximum in-plane shear stress and average normal stress at the point. Specify the orientation...
-
Determine the equivalent state of stress on an element at the point which represents (a) the principal stresses and (b) the maximum in-plane shear stress and the associated average normal stress....
-
Determine the equivalent state of stress on an element at the same point which represents (a) the principal stress, and (b) the maximum in-plane shear stress and the associated average normal stress....
-
f(x) = 9x +21x - 5x - 25 W Entry tip: Enter the zeros (exact values, not decimal approximations) separated by commas. Question Help: VIDEO Message instructor Submit Question Jump to Answer
-
Solve the equation: (x + 10) (3x-1) = - 7x - 118 x =
-
Consider the following quadratic equation: -3x+4x=-3 108:23:11 Step 1 of 2: Find the values of a, b, and c that should be used in the quadratic formula to determine the solution of the quadratic...

Study smarter with the SolutionInn App


