Consider 1,2,3-trichlorobenzene and 1,3,5-trichlorobenzene, both shown below (the carbon atoms are numbered as shown). Both are flat
Question:
Consider 1,2,3-trichlorobenzene and 1,3,5-trichlorobenzene, both shown below (the carbon atoms are numbered as shown). Both are flat molecules with 120° bond angles, yet one of them is polar and the other nonpolar. Which is which, and why? For the polar one, indicate which part of the molecule is δ+ and which is δ–.
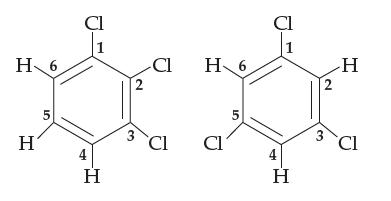
Transcribed Image Text:
H 6 H 5 Cl 1 H 2 3 -Cl Cl H 6 Cl 5 Cl H 2 3 H Cl
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
123trichlorobenzene is polar while 135trichlorobenzene is nonpolar To determine the polarity of a mo...View the full answer

Answered By

Mustafa olang
Please accept my enthusiastic application to solutionInn. I would love the opportunity to be a hardworking, passionate member of your tutoring program. As soon as I read the description of the program, I knew I was a well-qualified candidate for the position.
I have extensive tutoring experience in a variety of fields. I have tutored in English as well as Calculus. I have helped students learn to analyze literature, write essays, understand historical events, and graph parabolas. Your program requires that tutors be able to assist students in multiple subjects, and my experience would allow me to do just that.
You also state in your job posting that you require tutors that can work with students of all ages. As a summer camp counselor, I have experience working with preschool and kindergarten-age students. I have also tutored middle school students in reading, as well as college and high school students. Through these tutoring and counseling positions, I have learned how to best teach each age group. For example, I created songs to teach my three-year-old campers the camp rules, but I gave my college student daily quizzes to help her prepare for exams.
I am passionate about helping students improve in all academic subjects. I still remember my excitement when my calculus student received her first “A” on a quiz! I am confident that my passion and experience are the qualities you are looking for at solutionInn. Thank you so much for your time and consideration.
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
A local manufacturing company produces heating and colling appliances for home and industrial applications. These include refrigerators, water coolers, water heaters, air conditioners, sandwich...
-
Consider the tensile stress-strain diagrams in Figure 6-28 labeled 1 and 2 and answer the following questions. These diagrams are typical of metals. Consider each part as a separate question that has...
-
For each of the following organic molecules draw a Lewis structure in which the carbon atoms are bonded to each other by single bonds: (a) C2H6, (b) C4H10, (c) C5H12. For (b) and (c), show only...
-
The OrbitTrack Company specializes in developing and selling a wide range of high-quality scooters. Sales representatives report that there is a growing demand for racing scooters. OrbitTrack's...
-
Spaulding Manufacturing Company has determined the cost of manufacturing a unit of product as follows, based on normal production of 100,000 units per year: Direct materials . . . . . . . . . . . . ....
-
What is the primary difference between an annual bond and a semiannual bond? What changes do you need to make in finding the price of a semiannual bond versus an annual bond?
-
Identify the four components of an ecosystem. After you do this try to visualize the interactions of these four components of an ecosystem as illustrated by Figure 2. 2 in the textbook. Does this...
-
Christopher City received a contribution of $520,000 to provide scholarships to the children of deceased city employees. The donor stipulated that all income, including both realized and unrealized...
-
*7. Verify the divergence theorem (i.e. show in the mathematical statement of the theorem that LHS = RHS) for the vector field A = 2xzi+zxj + (z xyz + 2)k, : and the region in z 0 enclosed by x +...
-
Cyclohexane, formula C 6 H 12 , is often drawn as a puckered (not flat) ring as shown below (the purple and blue atoms are the hydrogens). Explain how drawing it as a puckered ring makes it...
-
There are exceptions to the predictions of VSEPR. Consider CH 3 , known as a methyl radical. (a) Create a dot diagram for the methyl radical. How is it fundamentally different from other dot diagrams...
-
Most adult Americans drive. But do you have any idea how many licensed drivers there are in each U.S. state? The table here lists the number of male and female drivers licensed in each of 15 randomly...
-
Describe the effects of a decrease in the interest rate on present and next periods consumption if the individual is a net lender (i.e., has savings) after period 1 and the substitution effect is...
-
Why does real wage rigidity contribute to unemployment? What are its causes?
-
What is the natural rate of unemployment? What has caused the natural rate to change over time?
-
What benefits does a credible nominal anchor provide?
-
Suppose you are about to buy a car and ask to see a vehicle history report to check on previous accidents or problems reported for that car. When you are told that this information is not available,...
-
Assume Casual Wear opened a store in San Francisco, starting with cash and common stock of $98,000. Nicole Marchildon, the store manager, then signed a note payable to purchase land for $94,000 and a...
-
l ask this second time correnct answer is 38,01 can we look pls Consider a non-conducting rod of length 8.8 m having a uniform charge density 4.5 nC/m. Find the electric potential at P, a...
-
Find the result of operating with (1/r 2 ) (d /dr) (r 2 d /dr) + 2/r on the function Ae br . What must the values of A and b be to make this function an eigenfunction of the operator?
-
Normalize the set of functions n (θ) = e inθ , 0 ¤ θ ¤ 2Ï. To do so, you need to multiply the functions by a so-called normalization constant...
-
Show that the set of functions n () = e in , 0 2is orthogonal if n is an integer. To do so, you need to 2 0 * m () n () = d = 0 for m n if n and m are intergers.
-
5. You have four successive polarizing filters. The first is horizontal, the second is 48 from vertical, the third is 63.7 from vertical and the last is vertical. What percentage of light is...
-
4. The electric range on your stove works by running high current (55 A at 240 V) through a large wire until it heats up and glows red. If you measure that glow to have a frequency of 413.5 THz, what...
-
A radio antenna broadcasts a 1.0 MHz radio wave with 22.0 kW of power. Assume that the radiation is emitted uniformly in all directions.

Study smarter with the SolutionInn App


