Consider the compounds titanium tetrachloride (TiCl 4 ) and zirconium tetrachloride (ZrCl 4 ). Zirconium is in
Question:
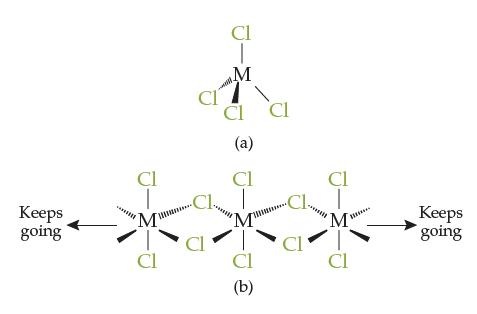
Consider the compounds titanium tetrachloride (TiCl4) and zirconium tetrachloride (ZrCl4). Zirconium is in the same group as titanium on the periodic table, and both Ti–Cl and Zr–Cl polar covalent bonds are quite strong. TiCl4 has a melting point of –25°C (i.e., it is a liquid at room temperature). ZrCl4 has a melting point of 437°C (i.e., it is a solid at room temperature). Based on this information, which diagram goes with which compound? Justify your explanation.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





